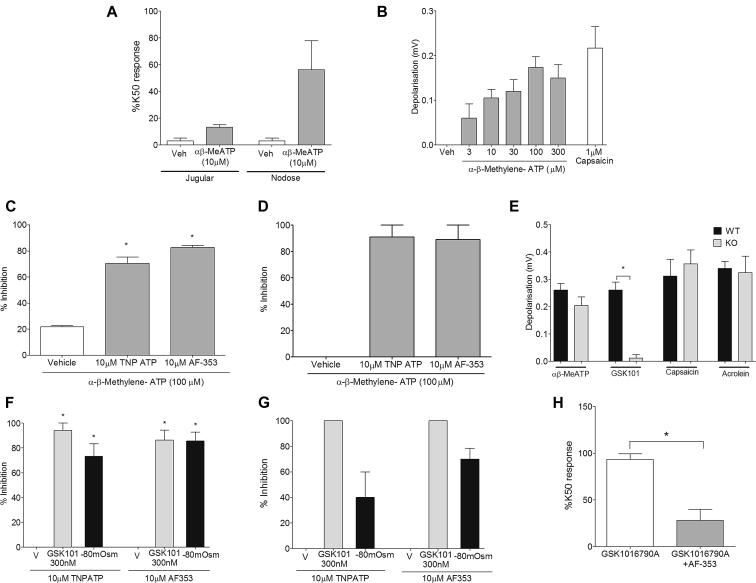
Fig 5.
A and B, αβ-MeATP (10 μmol/L) caused an increase in [Ca2+]i levels predominantly from guinea pig airway–specific nodose ganglion neurons (Fig 5, A) and a concentration-dependent depolarization of the guinea pig vagus nerve (Fig 5, B). C and D, This response was inhibited by the P2X3 antagonists AF-353 (10 μmol/L) and TNP-ATP (10 μmol/L) in both guinea pig (Fig 5, C) and donor human (Fig 5, D) vagus. E, Responses to GSK1016790A (300 nmol/L) were virtually abolished in Px1−/− mice. KO, Knockout; WT, wild-type. F and G, Both GSK1016790A- and −80 mOsm–induced depolarization in guinea pig (Fig 5, F) and donor human (Fig 5, G) vagus was inhibited by AF-353 (10 μmol/L) and TNP-ATP (10 μmol/L). H, [Ca2+]i signal induced by GSK1016790A (30 nmol/L) in the guinea pig airway–specific nodose ganglia neurons was also inhibited by AF-353 (10 μmol/L; N = 3; n = 4). Data are presented as means ± SEMs of 4 to 6 observations for guinea pig and 2 to 3 observations for human experiments. *Statistical significance (P < .05), unpaired t test comparing responses in Px1−/− vagus with wild-type control or GSK1016790A-induced [Ca2+]i responses in nodose neurons.