Figure 7.
Rif1’s Anaphase and S-Phase Functions Are Separate
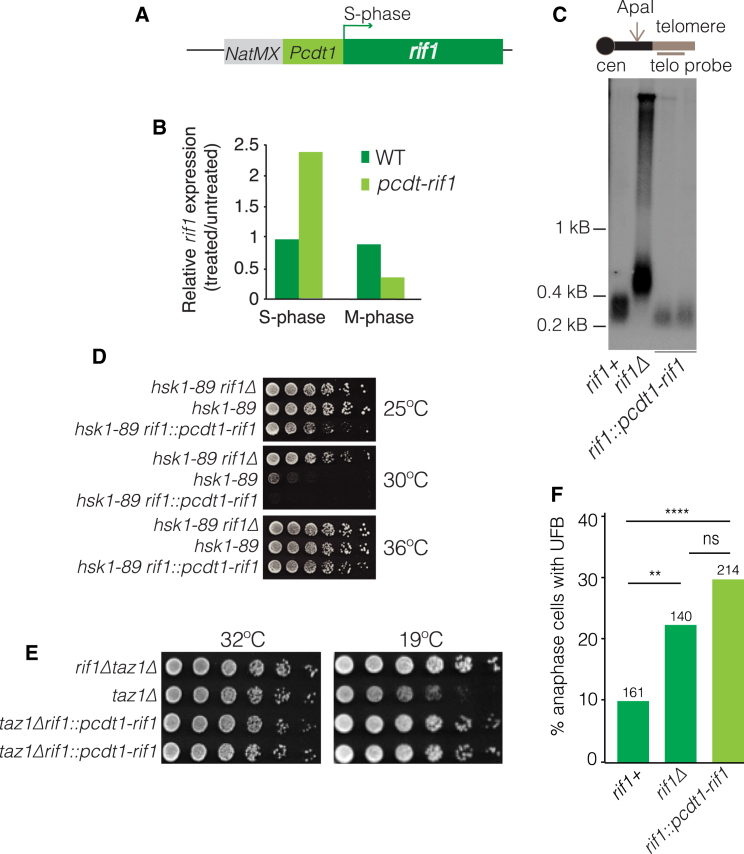
(A) Schematic of approach to induce S-phase-specific rif1+ expression. The cdt1+ promoter region (640 bp, including the binding site for the S-phase transcription factor Cdc10 and cdt1 transcription start site and 5′ UTR) was inserted immediately upstream of the endogenous rif1 ORF. NatMx provides a selectable marker.
(B) Relative rif1 transcript levels were determined by qRT-PCR and normalized to levels of act1. (S phase) Cells were grown in media containing 15 mM hydroxyurea (Sigma) for 4 hr at 32°C. (M phase) Cells were treated with 50 μg/ml TBZ (thiabendazole, a microtubule-depolymerizing agent) for 2 hr at 25°C. Arrest was verified via septation index.
(C) pcdt1-rif1 confers WT telomere length, confirming S-phase function. Southern blot as in Figure 4. Two individual clones of the pcdt1-rif1 strain were analyzed.
(D) pcdt1-rif1 confers WT Rif1 function with respect to Hsk1-mediated replication control. 5-fold serial dilutions of log phase cells at 25°C (permissive for hsk1-89) incubated at 25°C, 30°C, or 36°C. While rif1Δ restores growth of hsk1-89 cells at a non-permissive (30°C) temperature, pcdt1-rif1 behaves as rif1+ in failing to do so, confirming Rif1 functionality in S phase. Replicates grown at 36°C serve as loading control.
(E) Dilution assay as in (D). pcdt1-rif1 behaves as rif1Δ by rescuing taz1Δ cold sensitivity.
(F) Frequencies of cenII-tetO/R-tomato-UFBs are quantified as in Figure 6C. pcdt1-rif1 phenocopies rif1Δ in elevating UFB levels, tying UFB resolution to Rif1 function outside of S phase. ∗∗p < 0.01, ∗∗∗∗p < 0.0001.