Figure 1. Generation of a knock‐in (KI) mouse line carrying the mutation H191Q on the T‐type Cav3.2 channel .
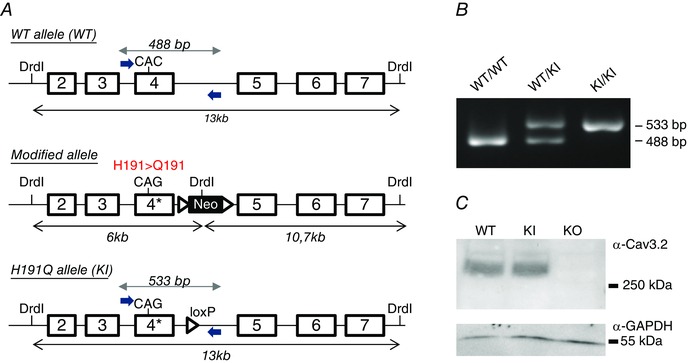
A, the Cav3.2 allele was created by homologous recombination (see Materials and Methods). The CAC codon encoding histidine 191 (H191) in exon 4 of the WT allele (upper panel) was replaced by the codon CAG encoding glutamine (Q), and a loxP‐flanked neo cassette was introduced 3′ from exon 4 to select for embryonic stem cells harbouring the knock‐in (KI) allele (middle panel). The final mutant allele was obtained after excision of the neo cassette by a Cre recombinase treatment of embryonic stem cells (lower panel). B, henotyping of the Cav3.2‐H191Q KI mice. PCR analysis using specific primers positioned in A revealed the WT allele (488 bp) in both WT and heterozygous animals as well as the mutant allele (533 bp) in both KI and heterozygous animals. The 45 bp difference between mutant and WT alleles results from the remaining loxP site in the mutant allele. C, representative Western‐blot analysis of the expression level of the Cav3.2 protein in the whole brain of WT and KI mice; a whole brain extract from knock‐out mice for Cav3.2 (KO) was used as a negative control in these experiments. The expression level of α‐GAPDH was used as loading control in these experiments (lower panel).
