Abstract
Zika virus (ZIKV) is causing an explosive outbreak of febrile disease in the Americas. There are no effective antiviral therapies or licensed vaccines for this virus, and mosquito control strategies have not been adequate to contain the virus. A promising candidate for arbovirus control and prevention relies on the introduction of the intracellular bacterium Wolbachia into Aedes aegypti mosquitoes. This primarily has been proposed as a tool to control dengue virus (DENV) transmission; however, evidence suggests Wolbachia infections confer protection for Ae. aegypti against other arboviruses. At present, it is unknown whether or not ZIKV can infect, disseminate, and be transmitted by Wolbachia-infected Ae. aegypti. Using Ae. aegypti infected with the wMel strain of Wolbachia that are being released in Medellin, Colombia, we report that these mosquitoes have reduced vector competence for ZIKV. These results support the use of Wolbachia biocontrol as a multivalent strategy against Ae. aegypti-transmitted viruses.
Zika virus (ZIKV) is an arbovirus that belongs to the family Flaviviridae. It currently is causing an explosive outbreak of febrile disease in the Americas. In humans, ZIKV infection typically causes a mild and self-limiting illness known as Zika fever, which often is accompanied by maculopapular rash, headache, and myalgia1,2. During the current outbreak, a causal relationship has been established between prenatal ZIKV infection and microcephaly and other serious brain anomalies3,4,5,6. Prior to this, ZIKV existed in relative obscurity with only sporadic confirmed human infections until the end of the last century7. The virus is believed to have originated in Africa, where it still circulates enzootically among unknown vertebrate hosts (presumably nonhuman primates), and is transmitted by arboreal Aedes mosquitoes8,9,10. These cycles lead to occasional outbreaks of spillover infection in Africa, but most human cases around the globe result from ZIKV emergence into a human-mosquito cycle involving Aedes aegypti11 and/or other urban or peri-urban Aedes species, e.g., Aedes albopictus and Aedes hensilli12,13,14.
Despite the continued spread of the virus, there remain no effective antiviral therapies or licensed vaccines. Thus, with its continued invasion of the new world, the only tools presently available to combat Zika target mosquito populations, mostly with insecticides and larval source reduction. To date, these strategies have not prevented invasion of this virus into new locales and have not been adequate to control the virus upon arrival. A promising candidate for arbovirus control and prevention relies on the introduction of the intracellular bacterium Wolbachia into Ae. aegypti mosquitoes. Wolbachia is a maternally-inherited intracellular bacterium that is present in numerous insect species worldwide, including mosquitoes, butterflies, beetles, ants, and bees15,16. Interestingly, Ae. aegypti has no native Wolbachia symbionts. The wMelPop strain of the bacterium therefore was introduced into Ae. aegypti with the intent to control dengue virus (DENV) transmission by shortening the lifespan of female mosquitoes17. This alone could have had a potentially major impact on disease transmission by greatly reducing the number of females old enough to transmit the virus18; however, that strain of Wolbachia no longer is being considered for biocontrol because infected mosquitoes displayed reduced fitness in small-scale field releases19. Serendipitously, it also was discovered that some Wolbachia interfere with viruses and other microbes in the same host. For example, certain Wolbachia variants (e.g., the wMel strain) partially block DENV transmission without greatly impacting Ae. aegypti fitness20,21,22. The wMel strain conferred protection against chikungunya virus (CHIKV) and yellow fever virus (YFV) in Ae. aegypti as well23,24,25. These findings lend optimism that Wolbachia biocontrol represents a significant new development in the fight against arbovirus transmission. Indeed, because DENV, CHIKV, YFV, and now ZIKV26,27,28 co-circulate in many parts of the tropics Wolbachia biocontrol could potentially be used as a multivalent strategy against all four of these Ae. aegypti-transmitted viruses. But the ability of ZIKV to infect, disseminate, and be transmitted by Wolbachia-infected Ae. aegypti has not been evaluated.
Accordingly, we assessed vector competence for ZIKV in wMel-infected and wMel-free Ae. aegypti from Medellin, Colombia. This was done because medium-scale deployments of wMel-infected Ae. aegypti began in the DENV metropolitan area of Medellin in the spring of last year (2015) [see www.eliminatedengue.com/colombia], and now ZIKV co-circulates with DENV in Colombia. The data presented herein provide information on the effectiveness of Colombian Wolbachia-infected Ae. aegypti in blocking the transmission of ZIKV, as well as describe a biologically relevant model for studying ZIKV transmission dynamics (i.e., exposure to virus was accomplished by feeding on a viremic host) that does not rely on animal blood spiked with cultured virus. These data argue for the expansion of this technology to ZIKV in South and Central America and are useful in the broader context of ZIKV-mosquito interactions in the Americas.
Results
Here, we verified that the phenotype of reduced vector competence existed in Wolbachia-infected laboratory colonies of Colombian Ae. aegypti for ZIKV. Adult, female, mosquitoes were exposed to infectious bloodmeals containing ZIKV and mosquitoes that ingested blood containing virus were assayed for infection, dissemination, and transmission potential at 4, 7, 10, 14, and 17 days (d) post feeding (PF). ZIKV infection status was confirmed by plaque assay to differentiate infectious from non-infectious virus. As expected, infection, dissemination, and transmission rates were high for WT exposed to blood containing ZIKV. In contrast, (COL)wMel that ingested blood containing ZIKV displayed poor peroral vector competence as compared to WT. In fact, there was a significant reduction (Exact Unconditional Test) in ZIKV infection status as compared to WT at all timepoints assayed and across replicates, and (COL)wMel remained non-infective over the duration of experimentation (Figs 1, 2, 3). Similar findings also were recently reported by Dutra et al.29.
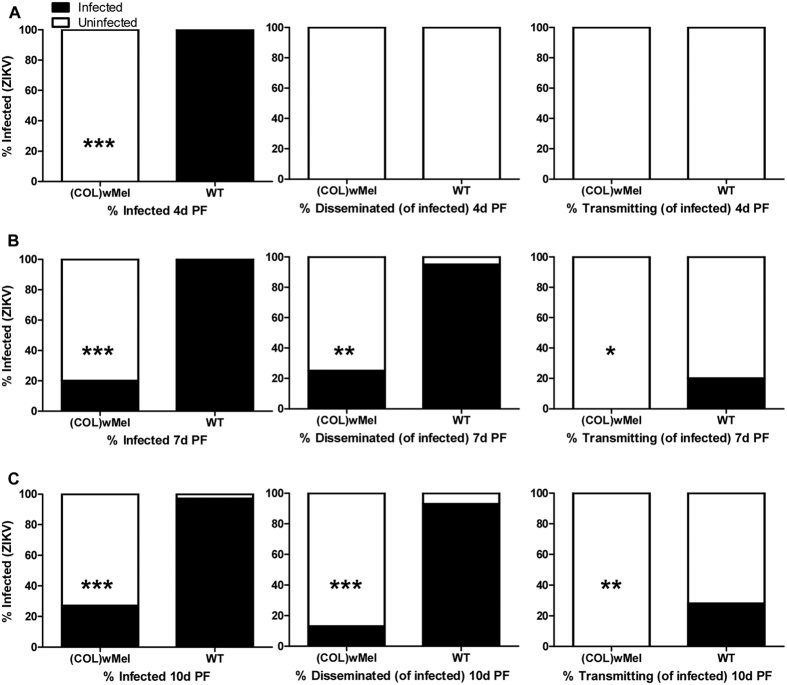
Figure 1. Vector competence of WT and (COL)wMel mosquitoes orally infected with 6.02 log10 PFU/ml of ZIKV.
Mosquitoes were allowed to feed on ZIKV-infected mice and were examined at days 4, 7, and 10 post feeding to determine infection, dissemination, and transmission efficiencies. Infection efficiency corresponds to the proportion of mosquitoes with virus-infected bodies among the tested ones. Dissemination efficiency corresponds to the proportion of mosquitoes with virus-infected legs, and transmission efficiency corresponds to the proportion of mosquitoes with infectious saliva among those infected. *significant reduction in infection rates (*p < 0.05, **p < 0.01, ***p < 0.001) (A). Four days post feeding (n = 20) (B). Seven days post feeding (n = 20) (C). Ten days post feeding (n = 30).
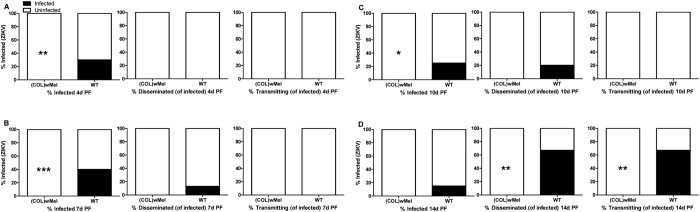
Figure 2. Vector competence of WT and (COL)wMel mosquitoes orally infected with 4.74 log10 PFU/ml of ZIKV.
Mosquitoes were allowed to feed on ZIKV-infected mice and were examined at days 4, 7, 10, and 14 post feeding to determine infection, dissemination, and transmission efficiencies. Infection efficiency corresponds to the proportion of mosquitoes with virus-infected bodies among the tested ones. Dissemination efficiency corresponds to the proportion of mosquitoes with virus-infected legs, and transmission efficiency corresponds to the proportion of mosquitoes with infectious saliva among those infected. *significant reduction in infection rates (*p < 0.05, **p < 0.01, ***p < 0.001) (A). Four days post feeding (n = 20) (B). Seven days post feeding (n = 20) (C). Ten days post feeding (n = 20) (D). Fourteen days post feeding (n = 20).
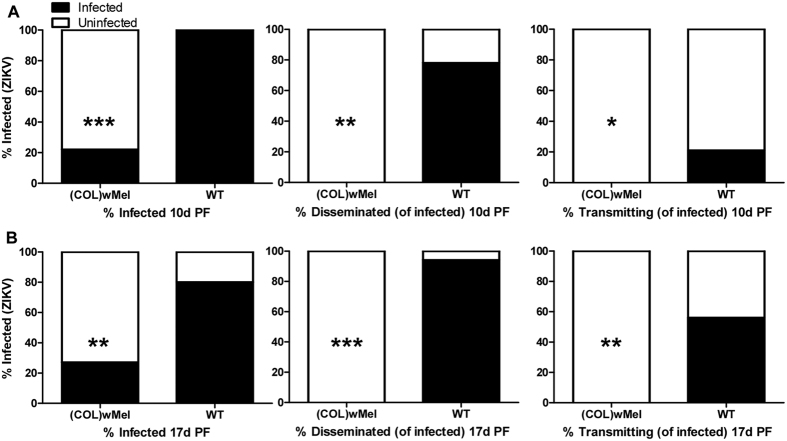
Figure 3. Vector competence of WT and (COL)wMel mosquitoes orally infected with 8.00 log10 PFU/ml of ZIKV.
Mosquitoes were exposed to a ZIKV-infected bloodmeal via water-jacketed membrane feeder and were examined at days 10 and 17 post feeding to determine infection, dissemination, and transmission efficiencies. Infection efficiency corresponds to the proportion of mosquitoes with virus-infected bodies among the tested ones. Dissemination efficiency corresponds to the proportion of mosquitoes with virus-infected legs, and transmission efficiency corresponds to the proportion of mosquitoes with infectious saliva among those infected. *significant reduction in infection rates (*p < 0.05, **p < 0.01, ***p < 0.001) (A). Ten days post feeding (n = 18 for (COL)wMel and n = 19 for WT) (B). Seventeen days post feeding (n = 15 for (COL)wMel and n = 20 for WT).
These data also are informative for a more accurate appraisal of ZIKV transmission by Ae. aegypti. To better understand ZIKV transmission dynamics, we assessed the subtleties of infection in WT over time following infectious bloodmeals that were in agreement with viremias detected in patients in Colombia where the mean ± standard deviation serum viral load was 4.42 log10 viral copies/ml ± 1.02 (n = 10, range = 2.73–5.84 log10 viral copies/ml). After feeding on ZIKV-infected mice with moderate viremia (6.02 log10 PFU/ml), 100% of WT mosquitoes had established infections at 4 d PF, but none of the mosquitoes disseminated virus nor was infectious virus detected in the saliva (Fig. 1A). At 7 d PF, most mosquitoes disseminated virus (95%) and a moderate number also were infective (Fig. 1B). By 10 d PF, the transmission rate had increased to 28% (Fig. 1C). In contrast, WT that fed on ZIKV-infected mice with low viremia (4.74 log10 PFU/ml) did not have detectable virus in the saliva until 14 d PF (Fig. 2A–C).
To investigate whether or not higher viral titers in the bloodmeal increase the probability of mosquito infection, mosquitoes were exposed to a relatively high viremic bloodmeal (8.00 log10 PFU/ml) via water-jacketed membrane feeder maintained at 36.5 °C30. Surprisingly, infection, dissemination, and transmission rates were almost identical for both (COL)wMel and WT exposed to viremic mice as compared to mosquitoes exposed to a membrane feeder spiked to a higher viremia (Figs 1 and 3). At 10 d PF, the infection rate for mouse-exposed (COL)wMel was 27% (Fig. 1C) and for membrane feeder-exposed (COL)wMel it was 22% (Fig. 3A). Likewise, the transmission rate for mouse-exposed WT was 28% and for membrane feeder-exposed WT it was 21%. By 17 d PF, the transmission rate peaked at 56% for membrane feeder-exposed WT (Fig. 3B). In comparison, we observed a transmission rate of 67% at 14 d PF in WT that were exposed to mice with relatively low viremias (Fig. 2C). These data highlight the importance of investigating vector competence by allowing mosquitoes to feed on a viremic host because membrane feeding influenced the magnitude of the observed effect.
Discussion
There is a paucity of data available on vector competence for ZIKV. The few studies available have primarily focused on two urban vectors, Ae. aegypti and Ae. albopictus13,31, but other Aedes species may be important vectors depending on the specific geographic location12,32,33. The first ZIKV vector competence study with Ae. aegypti was conducted in 195634 and it was demonstrated that Ae. aegypti was capable of transmitting ZIKV for ten weeks. Interestingly, ZIKV was not detectable on days 5 and 10 PF. Another study involving transmission to a rhesus monkey demonstrated that the extrinsic incubation period (EIP) of ZIKV was about 15 days35. Both of these results differ from what we report here. We were able to detect ZIKV infection in Ae. aegypti as early as 4 d PF and the EIP of ZIKV ranged from 7–14 days depending on viremia of the host. This may indicate differences between the propensity of African versus Asian lineage ZIKV to infect and replicate in Ae. aegypti but will require further validation in the laboratory.
More recently, it was demonstrated that Singapore’s Ae. aegypti were highly capable of transmitting ZIKV36. In contrast, Ae. aegypti strains from Kedougou and Dakar (Senegal) were not competent to transmit ZIKV32. Similar results recently were observed with Ae. aegypti strains from the Americas, which were susceptible to ZIKV infection but had unexpectedly low transmission potential31. Here we demonstrated that a strain of Ae. aegypti from Medellin, Colombia was highly susceptible to ZIKV infection and were competent in their potential to transmit the virus to a new host. It should be noted that Ae. aegypti with poor competence but high population density, have been capable of sustaining arbovirus outbreaks37. And, that the geographic variation in oral susceptibility of mosquitoes of the same species to different viruses is not unusual38,39,40. This argues for continued studies (both experimental and epidemiological) assessing interactions between differing Ae. aegypti-ZIKV combinations.
The history of ZIKV has been reminiscent of CHIKV, which re-emerged out of Africa and now circulates on all inhabited continents and represents a major global health problem. Not surprisingly, ZIKV has emerged in Colombia2, likely following the path of CHIKV, which reached the country in August 201441 and now both viruses co-circulate with DENV and YFV. Current vector control measures have been insufficient in preventing invasion of ZIKV and CHIKV into the country or controlling it after invasion. Although primarily deployed as a biocontrol tool for DENV, evidence suggests that Wolbachia can limit infection in Ae. aegypti with other arboviruses24,25; therefore, Wolbachia-infected Ae. aegypti could potentially be used to simultaneously control DENV, CHIKV, YFV, and ZIKV. As a result, we evaluated whether Colombian mosquitoes infected with the wMel strain of Wolbachia reduced ZIKV transmission potential. Studies like this one, aimed at assessing the potential effectiveness of Wolbachia biocontrol against ZIKV, will help inform the viability of using this technology as a multivalent strategy for multiple Ae. aegypti-transmitted arboviruses. This novel approach is already being tested in five countries around the globe (Australia, Brazil, Colombia, Indonesia, and Vietnam) against related arboviruses, so these results warrant further exploration, both in the laboratory and the field, on the feasibility of expanding this technology beyond DENV and informing whether Wolbachia biocontrol can be used to supplement or replace existing vector control strategies.
Methods
Ethics Statement
This study was carried out in strict accordance with recommendations set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals and animal facilities were under the control of the School of Veterinary Medicine with oversight from the University of Wisconsin Research Animal Resource Center. The protocol was approved by the University of Wisconsin Animal Care and Use Committee (Approval #V01327).
Cells and viruses
African Green Monkey kidney cells (Vero; ATCC #CCL-81) were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and incubated at 37 °C in 5% CO2. Aedes albopictus mosquito cells, (C6/36; ATCC #CRL-1660) were maintained in MEM supplemented with 10% FBS, 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 0.1 mM non-essential amino acids, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and incubated at 28 °C in 5% CO2. ZIKV strain PRVABC59 (GenBank:KU501215), originally isolated from a traveler to Puerto Rico with three rounds of amplification on Vero cells, was obtained from Brandy Russell (CDC, Ft. Collins, CO). Virus stocks were prepared by inoculation onto a confluent monolayer of C6/36 mosquito cells.
Mosquito strains and colony maintenance
Ae. aegypti used in this study were maintained at the University of Wisconsin-Madison as previously described42. Two lines of mosquitoes were used in this study. Wild type (WT) mosquitoes (not infected with Wolbachia) were established from several hundred eggs collected from 41 ovitraps placed around the municipality of Bello (commune 1), a northwest suburb of Medellin, Colombia. The Wolbachia-infected ((COL)wMel; infected with the wMel strain of Wolbachia pipientis) mosquito line was generated by crossing wild-caught Aedes aegypti with a wMel-infected laboratory strain of Ae. aegypti using a scheme of mating to field-collected males as developed by Yeap et al.43 to generate a Colombian genetic background Wolbachia-infected mosquito. The wMel-infected laboratory population of Ae. aegypti originated from eggs provided by Scott O’Neill (Monash University, Victoria Australia). Briefly, females from the wMel-infected laboratory population were backcrossed to wild-caught males until generation five at which time the lines were closed (restricting matings to mosquitoes from within the line) with the population size maintained at several thousand adults. An outcrossed line then was established by continuous backcrossing laboratory mosquitoes to the progeny of wild-caught Ae. aegypti from the Bello region (same location as eventual release zones). The WT line was derived from material collected from the same 41 ovitraps distributed throughout the suburb of Bello, Colombia. This line has never had any previous contact with Wolbachia-infected mosquitoes. Wild-caught males from the Bello region were routinely collected and introduced into the (COL)wMel and WT colony cages after each generation to prevent inbreeding effects and to ensure the genetic homogeneity of these two lines. Wolbachia infection status was routinely verified using PCR with primers specific to the IS5 repeat element20.
Exposure to infective bloodmeal
Mosquitoes were exposed to ZIKV by feeding on isoflurane anesthetized ZIKV-infected Ifnar−/− mice. These mice have abrogated type I interferon signaling and as a result develop lethal infection and a high viremia. Ifnar−/− mice on the C57BL/6 background were obtained from Eva Harris (University California-Berkeley, Berkeley, CA) and were bred in the pathogen-free animal facilities of the University of Wisconsin-Madison School of Veterinary Medicine. Groups of three-week-old mixed sex mice were used for mosquito exposures. Mice were infected in the left, hind foot pad with 106 plaque forming units (PFU) of ZIKV in 50 μl of animal diluent (AD: 1% heat-inactivated FBS in Dulbecco’s PBS). Uninfected mosquitoes (both WT and (COL)wMel) were allowed to feed on mice two or three days post infection at which time sub-mandibular blood draws were performed and serum was collected to verify viremia. Mice fed upon two days post infection (biological replicate one) yielded an infectious bloodmeal concentration of 6.02 log10 PFU/ml ± 0.67 (mean ± standard deviation; n = 4) and mice fed upon three days post infection (biological replicate two) yielded an infectious bloodmeal concentration of 4.74 log10 PFU/ml ± 0.06. A third biological replicate was performed, whereby mosquitoes were exposed to a ZIKV-infected bloodmeal via water-jacketed membrane feeder maintained at 36.5 °C30. Bloodmeals consisted of defibrinated sheep blood (HemoStat Laboratories) and fresh virus supernatant, yielding an infectious bloodmeal concentration of 8.0 log10 PFU/ml (bloodmeal titer was determined after feeding).
Vector Competence
Infection, dissemination, and transmission rates were determined for individual mosquitoes and sample sizes were chosen using long established procedures25,44,45. Briefly, three- to six-day-old female mosquitoes were sucrose starved for 14 to 16 hours prior to exposure to mice or membrane feeder. Mosquitoes that fed to repletion were randomized, separated into cartons in groups of 20–30, and maintained on 0.3 M sucrose in an environmental chamber at 26.5 °C ± 1 °C, 75% ± 5% relative humidity, and with a 12 hour photoperiod within the Department of Pathobiological Sciences BSL3 Insectary facility at the University of Wisconsin-Madison. All samples were screened by plaque assay on Vero cells. Dissemination was indicated by virus-positive legs. Transmission was defined as release of infectious virus with salivary secretions, i.e., the potential to infect another host, and was indicated by virus positive-salivary secretions.
Viral Quantification
All ZIKV screens from mosquito tissues and titrations for virus quantification from mouse serum or virus stocks were completed by plaque assay on Vero cell cultures. Duplicate wells were infected with 0.1 ml aliquots from serial 10-fold dilutions in growth media and virus was adsorbed for one hour. Following incubation, the inoculum was removed, and monolayers were overlaid with 3 ml containing a 1:1 mixture of 1.2% oxoid agar and 2X DMEM (Gibco, Carlsbad, CA) with 10% (vol/vol) FBS and 2% (vol/vol) penicillin/streptomycin. Cells were incubated at 37 °C in 5% CO2 for four days for plaque development. Cell monolayers then were stained with 3 ml of overlay containing a 1:1 mixture of 1.2% oxoid agar and 2X DMEM with 2% (vol/vol) FBS, 2% (vol/vol) penicillin/streptomycin, and 0.33% neutral red (Gibco). Cells were incubated overnight at 37 °C and plaques were counted. Viral RNA from human serum samples from Colombia was quantified by qRT-PCR using the primers and probe designed by Lanciotti et al.46. The RT-PCR was performed using the iTaqTM Universal One-Step RT-qPCR kit (BioRad, Hercules, CA) on an iCycler® instrument (BioRad, Hercules, CA). Primers and probe were used at final concentrations of 600 nm and 100 nm respectively. Cycling conditions were as follows: 37 °C for 15 min, 50 °C for 30 min and 95 °C for 2 min, followed by 50 cycles of 95 °C for 15 sec and 60 °C for 1 min. Virus concentration was determined by interpolation onto an internal standard curve made up of a 7-point dilution series of in vitro transcribed RNA.
Statistical Analysis
Infection, dissemination, and transmission rates were analyzed using an Exact unconditional test47. This test replaces Fisher’s exact test. It also is exact but has the advantage of being more sensitive in detecting differences (i.e., its statistical power is higher) in the case of sample sizes less than 10047. Each biological replicate consisted of mosquitoes from distinct generations to take into account stochastic variations.
Additional Information
How to cite this article: Aliota, M. T. et al. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci. Rep. 6, 28792; doi: 10.1038/srep28792 (2016).
Acknowledgments
The authors thank Emma Walker and Katrina Larkin for time spent rearing mosquitoes. We also thank the Eliminate Dengue Program for sharing Wolbachia-infected mosquitoes. This work was partially funded by National Institutes of Health Grant # R21 AI117413-01.
Footnotes
Author Contributions Conceived and designed the experiments: M.T.A., I.D.V. and J.E.O. Performed the experiments: M.T.A. and S.A.P. Analyzed the data: M.T.A. Contributed reagents/materials/analysis tools: M.T.A., J.E.O. and I.D.V. Wrote the paper: M.T.A.
References
- Campos G. S., Bandeira A. C. & Sardi S. I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 21, 1885–1886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho E., Paternina-Gomez M., Blanco P. J., Osorio J. E. & Aliota M. T. Detection of Autochthonous Zika Virus Transmission in Sincelejo, Colombia. Emerg. Infect. Dis. J. 22, 927–929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer O. Zika virus spreads across Americas as concerns mount over birth defects. BMJ 351, h6983 (2015). [DOI] [PubMed] [Google Scholar]
- Mlakar J. et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 0, null (2016). [DOI] [PubMed] [Google Scholar]
- Driggers R. W. et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 0, null (2016). [DOI] [PubMed] [Google Scholar]
- Rasmussen S. A., Jamieson D. J., Honein M. A. & Petersen L. R. Zika Virus and Birth Defects — Reviewing the Evidence for Causality. N. Engl. J. Med. 0, null (2016). [DOI] [PubMed] [Google Scholar]
- Hayes E. B. Zika virus outside Africa. Emerg. Infect. Dis. 15, 1347–1350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A. J., Williams M. C., Woodall J. P., Simpson D. I. & Goma L. K. Twelve isolations of zika virus from aedes (stegomyia) africanus (theobald) taken in and above a uganda forest. Bull. World Health Organ. 31, 57–69 (1964). [PMC free article] [PubMed] [Google Scholar]
- McCrae A. W. & Kirya B. G. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans. R. Soc. Trop. Med. Hyg. 76, 552–562 (1982). [DOI] [PubMed] [Google Scholar]
- Wolfe N. D. et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am. J. Trop. Med. Hyg. 64, 310–316 (2001). [DOI] [PubMed] [Google Scholar]
- Marchette N. J., Garcia R. & Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 18, 411–415 (1969). [DOI] [PubMed] [Google Scholar]
- Duffy M. R. et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 360, 2536–2543 (2009). [DOI] [PubMed] [Google Scholar]
- Wong P.-S. J., Li M. I., Chong C.-S., Ng L.-C. & Tan C.-H. Aedes (Stegomyia) albopictus (Skuse): A Potential Vector of Zika Virus in Singapore. PLoS Negl Trop Dis 7, e2348 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grard G. et al. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 8, e2681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H. Biology of Wolbachia. Annu. Rev. Entomol. 42, 587–609 (1997). [DOI] [PubMed] [Google Scholar]
- Zug R. & Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PloS One 7, e38544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman C. J. et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323, 141–144 (2009). [DOI] [PubMed] [Google Scholar]
- Rasgon J. L. & Scott T. W. Impact of population age structure on Wolbachia transgene driver efficacy: ecologically complex factors and release of genetically modified mosquitoes. Insect Biochem. Mol. Biol. 34, 707–713 (2004). [DOI] [PubMed] [Google Scholar]
- Nguyen T. H. et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasit. Vectors 8, 563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453 (2011). [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A. et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 (2011). [DOI] [PubMed] [Google Scholar]
- Frentiu F. D. et al. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl Trop Dis 8, e2688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009). [DOI] [PubMed] [Google Scholar]
- van den Hurk A. F. et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 6, e1892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota M. T. et al. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 10, e0004677 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S. & Valadere A. M. Transcontinental movement of Asian genotype chikungunya virus. Emerg. Infect. Dis. 20, 1400–1402 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D. Zika Virus Transmission from French Polynesia to Brazil. Emerg. Infect. Dis. 21, 1887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D., Cao-Lormeau V. M. & Gubler D. J. Zika virus: following the path of dengue and chikungunya? Lancet Lond. Engl. 386, 243–244 (2015). [DOI] [PubMed] [Google Scholar]
- Dutra H. L. et al. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host & Microbe 19(6), 771–774 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge L. C., Ward R. A. & Gould D. J. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq. News 24, 407–419 (1964). [Google Scholar]
- Chouin-Carneiro T. et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLOS Negl Trop Dis 10, e0004543 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagne C. T. et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect. Dis. 15, 492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G. W. A., Kitchen S. F. & Haddow A. J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520 (1952). [DOI] [PubMed] [Google Scholar]
- Boorman J. P. & Porterfield J. S. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans. R. Soc. Trop. Med. Hyg. 50, 238–242 (1956). [DOI] [PubMed] [Google Scholar]
- Cornet M., Robin Y., Adam C., Valade M. & Calvo M.-A. Comparison between experimental transmission of yellow fever and Zika viruses in Aedes aegypti. Cah Orstom Ser Entomol Med Parasitol 27, 47–53 [Google Scholar]
- Li M. I., Wong P. S. J., Ng L. C. & Tan C. H. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl. Trop. Dis. 6, e1792 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. R., Monath T. P., Tabachnick W. J. & Ezike V. I. Epidemic yellow fever caused by an incompetent mosquito vector. Trop. Med. Parasitol. Off. Organ Dtsch. Tropenmedizinische Ges. Dtsch. Ges. Für Tech. Zusammenarbeit GTZ 40, 396–399 (1989). [PubMed] [Google Scholar]
- Gubler D. J. & Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with dengue viruses. Am. J. Trop. Med. Hyg. 25, 318–325 (1976). [DOI] [PubMed] [Google Scholar]
- Gubler D. J., Nalim S., Tan R., Saipan H. & Sulianti Saroso J. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am. J. Trop. Med. Hyg. 28, 1045–1052 (1979). [DOI] [PubMed] [Google Scholar]
- Lambrechts L. et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol. Biol. 9, 160 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar S. et al. Outbreak of Chikungunya virus in the north Caribbean area of Colombia: clinical presentation and phylogenetic analysis. J. Infect. Dev. Ctries. 9, 1126–1132 (2015). [DOI] [PubMed] [Google Scholar]
- Christensen B. M. & Sutherland D. R. Brugia pahangi: Exsheathment and Midgut Penetration in Aedes aegypti. Trans. Am. Microsc. Soc. 103, 423–433 (1984). [Google Scholar]
- Yeap H. L. et al. Dynamics of the ‘popcorn’ Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187, 583–595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota M. T. et al. Characterization of Rabensburg virus, a flavivirus closely related to West Nile virus of the Japanese encephalitis antigenic group. PloS One 7, e39387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger-Lucarelli J. et al. Dissecting the role of E2 protein domains on alphavirus pathogenicity. J. Virol. doi: 10.1128/JVI.02792-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S. et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 14, 1232–1239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiczigel J., Abonyi-Tóth Z. & Singer J. An exact confidence set for two binomial proportions and exact unconditional confidence intervals for the difference and ratio of proportions. Comput. Stat. Data Anal. 52, 5046–5053 (2008). [Google Scholar]