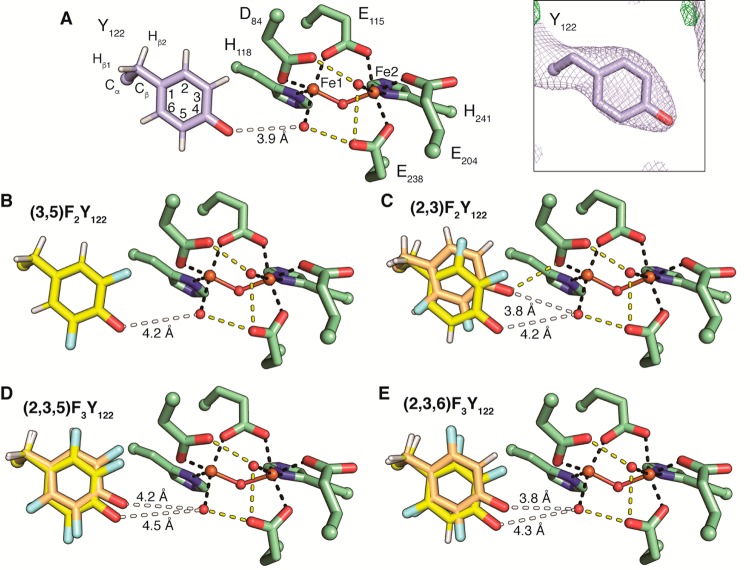
Figure 2.
Cofactor arrangement in FnY-β2. (A) The structure of wild-type met-β2 contains a diferric cluster (Fe1 and Fe2, ball and stick) that coordinates two water molecules (red spheres). Iron ligation by protein residues (green) or water is represented by black dashes. Putative hydrogen bonds are shown as yellow dashes. Y122 (light blue) is positioned adjacent to Fe1, but not within hydrogen bonding distance (white dash). The electron density for Y122 is shown inset (2Fo – Fc at 1 σ; green: Fo – Fc at 3 σ; red: Fo – Fc at −3 σ). (B–E) Each FnY-β2 is shown as in (A). For (2,3)F2Y, (2,3,5)F3Y, and (2,3,6)F3Y two conformations are present. The IN conformation (yellow) in each structure places the fluorine atoms on carbons 2 and 3 adjacent to D84. The OUT conformation (orange) is flipped 180°. Analysis of the occupancy of these conformations (Figure S6) suggests the IN conformation is typically dominant.