Abstract
The chaperome constitutes a broad family of molecular chaperones and co-chaperones that facilitate the folding, refolding, and degradation of the proteome. Heat shock protein 90 (Hsp90) promotes the folding of numerous oncoproteins to aid survival of malignant phenotypes, and small molecule inhibitors of the Hsp90 chaperone complex offer a viable approach to treat certain cancers. One therapeutic attribute of this approach is the selectivity of these molecules to target high affinity oncogenic Hsp90 complexes present in tumor cells, which are absent in nontransformed cells. This selectivity has given rise to the idea that disease may contribute to forming a stress chaperome that is functionally distinct in its ability to interact with small molecule Hsp90 modulators. Consistent with this premise, modulating Hsp90 improves clinically relevant endpoints of diabetic peripheral neuropathy but has little impact in nondiabetic nerve. The concept of targeting the “diabetic chaperome”to treat diabetes and its complications is discussed.
Keywords: Bioenergetics, Heat shock proteins, Inflammation, Molecular chaperones, Neurodegeneration, Oxidative stress
Introduction
The 2014 National Diabetes Statistics Report estimates that 29.1 million people in the USA have diabetes (inclusive of 8.1 million undiagnosed patients; http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html). Despite the use of insulin, incretin mimetics, and various oral antidiabetic medications to help maintain euglycemia, many of these individuals develop diabetic peripheral neuropathy (DPN) [1]. Diabetes often leads to the development of a distal symmetric sensorimotor polyneuropathy that typically presents as a “stocking-glove”change in sensation. This change in sensation is due to neurodegeneration that initiates at the distal ends of axons within the legs and arms and progresses proximally. Sensory symptoms often predominate early in the disease and may manifest as a painful and/or insensate neuropathy associated with dysfunction and loss of small thinly myelinated or unmyelinated sensory fibers. More progressive disease can impact motor fibers, which contributes to losses in vibratory sensation, proprioception, decreased nerve conduction velocity, and eventually, irreversible neurodegeneration [2].
Considerable progress has been made in understanding the pathogenesis of DPN. Molecular targets that are relatively “diabetes specific”(polyol and hexosamine pathways, advanced glycation end products) or which are altered in numerous disease states (PKC activation, decreased neurotrophic support, enhanced oxidative stress) contribute to the progressive degeneration of small and large sensory fibers that underlies painful and insensate DPN [3]. Though FDA-approved options exist to treat painful DPN, they are less than optimal [4]. Unfortunately for patients with insensate DPN, progress toward understanding disease pathogenesis has not yielded any robust therapeutics to aid its management. Although minimizing oxidative stress with α-lipoic acid shows a limited benefit in improving some symptoms of insensate DPN [5–7], neither small molecule inhibitors of these pathways nor growth factor therapies have met with translational success [8]. One difficulty associated with the pharmacological management of DPN is that the contribution of these targets/pathways to disease symptoms does not necessarily occur with biochemical and/or temporal equivalence between patients over the typical history of the disease. Thus, pharmacologic approaches that are relatively insensitive to underlying pathogenic mechanisms may afford a novel disease-modifying approach to improve nerve function by helping cells tolerate diabetic stress in the face of recurring hypoglycemic and hyperglycemic swings [9].
Many neurodegenerative diseases can be considered protein-conformation disorders since their etiology is linked to the accumulation of mis-folded or aggregated proteins (β-amyloid and tau in Alzheimer’s disease, α-synuclein in Parkinson’s disease). Although the etiology of DPN is not linked to the accumulation of a specific mis-folded or aggregated protein, hyperglycemic stress can increase oxidative modification of proteins that can damage protein structure, impair protein folding, decrease refolding of damaged proteins, and/or induce protein aggregation. Moreover, postmitotic neurons and myelinated Schwann cells are very sensitive to mis-folded or damaged proteins when clearance mechanisms are compromised [10–12]. Endogenously, the cellular route to regulate mis-folded or damaged proteins is via interactions with members of the cellular chaperome.
The chaperome [13] represents the broad contingent of individual molecular chaperones and chaperone complexes that are expressed under normal proteostasis as well as proteotoxic conditions related to disease progression [14, 15]. Molecular chaperones such as heat shock protein 90 (Hsp90) and Hsp70 work in concert with a host of co-chaperones to fold nascent polypeptides into their final biologically active conformations. They also aid the refolding of aggregated and denatured proteins, and direct proteins toward degradation via the proteasome or by chaperone-mediated autophagy [16, 17]. Although changes in the chaperome have not been identified as essential to the development of diabetes and its complications, emerging evidence supports that pharmacologic modulation of the chaperome provides a powerful approach to improve insulin resistance [18] and diabetic complications such as nephropathy [19•, 20] and peripheral neuropathy [3]. Moreover, it is becoming quite clear that the drug-response phenotype to small molecule Hsp90 modulators can be influenced by disease-induced changes in the composition of chaperone complexes [21]. Therefore, the goals of this review are to highlight how pharmacologic modulation of the chaperome may improve DPN and consider whether diabetes-induced changes in the chaperome may influence the efficacy and selectivity of a promising class of therapeutics, C-terminal Hsp90 modulators.
Defining the Chaperome and Its Functions
Molecular chaperones are often referred to as heat shock proteins despite many members of this protein class not being induced by heat shock or other stress [22]. Molecular chaperones are commonly categorized based upon their molecular mass and may contain several isoforms. For example, the Hsp90 family contains four isoforms: inducible cytosolic Hsp90α, constitutive cytosolic Hsp90β, the endoplasmic reticulum localized glucose regulated protein 94 (Grp94, endoplasmin, Hsp90b1), and the mitochondrial tumor necrosis factor receptor type 1-associated protein (TRAP1, Hsp75). The Hsp70 family is much larger and is composed of 17 genes and 13 proteins [23, 24]. The more investigated forms comprise the constitutively expressed Hsc70, inducible Hsp72 (Hsp70 in mouse), the endoplasmic reticulum localized Grp78 (BiP, Hsp70-5), and the mitochondrial Grp75 (mortalin, mtHsp70, stress protein-70). Other members of the chaperome include small Hsps such as Hsp27, co-chaperones such as Hsp110 and Hsp40 (a family of DnaJ proteins [25]), and cytosolic and mitochondrial (Hsp60, Hsp10) chaperonins. Approximately 147 chaperones, co-chaperones, nucleotide exchange factors, and folding enzymes such as peptidyl-prolyl and protein disulfide isomerases (immunophilins) form the human chaperome [22]. As a group, these proteins are eight times more abundant than nonchaperome proteins in HeLa cells, and it is estimated that intracellular Hsp90 and Hsp70 isoforms may comprise 5 % (300–350 g) of total intracellular protein in an unstressed adult human [22].
Through its intrinsic ATPase activity and interaction with co-chaperones, Hsp90 plays a critical role in mediating protein folding [17, 25, 26]. However, Hsp90 is also a direct regulator of the heat shock response (HSR) since it binds to the transcription factor, heat shock factor 1 (HSF1), and suppresses its transactivating capacity [27, 28]. Upon exposure to numerous forms of cell stress, HSF1 dissociates, undergoes trimerization, and enters the nucleus to transcriptionally induce the synthesis of antioxidant proteins and chaperones, such as Hsp70. The induction of Hsp70 upon proteotoxic stress is well recognized as an adaptive response that aids the refolding and/or clearance of damaged or mis-folded proteins.
Disruption of the Hsp90/HSF1 complex can also be achieved pharmacologically with small molecules. Numerous small molecules have been demonstrated to modulate the interaction of Hsp90 with HSF1, induce Hsp70, and improve neuronal function [11, 29–35]. However, despite the fact that many of these small molecules interact with a similar region on Hsp90, they do not necessarily share a similar drug-response phenotype [14].
Pharmacologic Targeting of Hsp90—the Fit and the Form Begets the Function
Hsp90 contains N- and C-terminal nucleotide-binding domains that are joined by a charged, flexible middle domain capable of binding co-chaperones and client proteins [36]. While the C-terminal domain is essential for forming the functional Hsp90 homodimer, the N-terminal ATPase is necessary for the protein’s chaperone activity [36, 37]. However, Hsp90 does not work alone but rather as a component of a heteroprotein complex that directs protein folding. Briefly, Hsp70 recognizes surface accessible hydrophobic motifs in newly translated or damaged proteins [38], and in conjunction with a DnaJ protein [25], forms a complex with Hsp90 and HOP (Hsp70/Hsp90-organizing protein). Upon transfer of the substrate to one Hsp90 protomer, ATP promotes N-terminal dimerization, forming a closed conformation that clamps the client protein. Co-chaperones, including immunophilins, p23, and especially Aha1 (ATPase homologue 1), enhance the weak intrinsic ATPase activity of Hsp90 to drive conformational folding and release of the client [37].
N-terminal Inhibitors
Numerous structurally distinct N-terminal Hsp90 inhibitors have been designed that target the ATP-binding pocket. These compounds block the protein’s chaperone activity and cause premature release of the client protein [37, 39]. As many Hsp90 client proteins are oncogenic, release of the unfolded oncoprotein promotes its degradation and leads to tumor cytotoxicity. However, the interaction of N-terminal inhibitors with the Hsp90 complex is affected by several factors that can alter the biologic response to the xenobiotic [14].
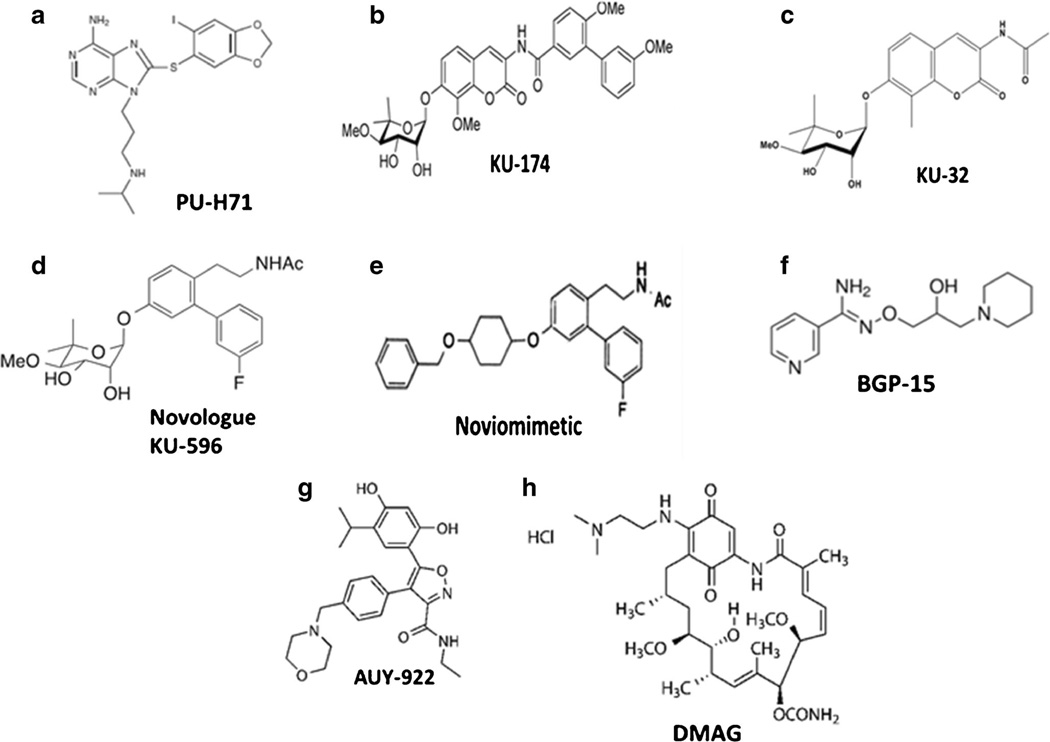
An attractive attribute of many N-terminal Hsp90 inhibitors is their selectivity toward targeting highly mitotic cancer cells with little effect on normal cell populations. This may be due to the presence of high affinity Hsp90 complexes that are present in cancer cells but are lacking in normal cells [40]. Consistent with this premise, cancer cells were found to express a mixture of low affinity, housekeeping Hsp90 species, and high affinity, stress-specific Hsp90 complexes [21]. Moreover, differences in the constitution of these stress-induced chaperone complexes may affect drug selectivity as well. For example, the purine-based N-terminal inhibitor, PU-H71 (Fig. 1a), pulled down Hsp90, co-chaperones, and client proteins that were not co-precipitated by other Hsp90 inhibitors [21, 41]. These data support the concept that disease can alter the composition of the Hsp90 complex and influence the binding of N-terminal inhibitors [14]. Further, stress-induced posttranslational modifications of Hsp90 can influence its interactions with N-terminal inhibitors [41, 42•]. For example, the interaction of PU-H71 with Hsp90 is less affected by phosphorylation of the protein compared to the prototypical N-terminal inhibitor, geldanamycin [41]. N-terminal Hsp90 inhibitors can also show a preferential interaction with SUMOylated versus non-SUMOylated Hsp90, resulting in greater activation of a HSR [42•]. Lastly, drug efficacy may also be influenced by the isoform ratio of chaperone complexes since N-terminal inhibitors can bind Hsp90β with greater affinity than Hsp90α [43]. Collectively, these data raise the possibility that the onset of disease may increase or possibly negate the therapeutic potential of various inhibitors by altering their recognition or interaction with Hsp90 isoforms and their heteroprotein complexes.
Fig. 1.
Structure of select Hsp90 modulators
Despite the observed selectivity for targeting stress-induced chaperone complexes to promote cytoxicity, a problematic issue with N-terminal Hsp90 inhibitors is the induction of the HSR. As induction of the HSR induces cytoprotective chaperones, this facilitates oncoprotein folding thereby diminishing toxicity, not a desired side effect for treating malignancies. On the other hand, promoting the HSR can facilitate the clearance of abnormally folded proteins, and N-terminal inhibitors have shown promise for treating neurodegenerative diseases whose etiology is associated with protein folding disorders [32, 35, 44–46]. However, the distribution of Hsp90α and Hsp90β isoforms and their differential interaction with various N-terminal inhibitors may also affect the robustness of the HSR. For example, compared to Hsp90β, Hsp90α binds HSF1 with greater affinity, and upon heat shock, this complex may not increase the expression of Hsp70 and Hsp40 as readily as the Hsp90β complex [43].
Clearly, N-terminal Hsp90 inhibitors possess both strengths and weaknesses as therapeutics, and this drug class has seen considerable clinical development for treating various forms of cancer [39, 47]. Though less evolved from a pharmacologic and therapeutic standpoint, compounds that target the C-terminal domain of Hsp90 may circumvent some of these issues and provide a novel path forward to treat cancer [48–51], neurodegeneration [52, 53], and DPN [3].
C-terminal Inhibitors
In contrast to N-terminal inhibitors, C-terminal inhibitors prevent formation of the Hsp90 homodimer and prohibit occupation and clamping of client proteins in the N-terminal binding pocket [54–56]. Several structurally distinct C-terminal inhibitors have been identified, and those based on the novobiocin scaffold have yielded two distinct inhibitor classes that have circumvented some of the issues observed with the N-terminal inhibitors [37]. As exemplified by KU-174 (Fig. 1b), the first class is highly cytotoxic and promotes client protein degradation at drug concentrations that do not increase the HSR [50, 51]. Similar to N-terminal inhibitors, KU-174 was selectively toxic to a prostate cancer cell line compared to normal human renal proximal tubule epithelial cells [48]. Whether this selectivity is related to disrupting cancer-specific chaperone complexes needs to be clarified. However, structure-activity studies have identified that the noviose sugar is critical for interacting with the C-terminal domain of Hsp90, while the biaryl side chain binds to Aha 1 and prevents its interaction with Hsp90 [57]. Since Aha 1 increases the chaperone’s ATPase activity, disrupting its association with Hsp90 leads to client protein degradation [57]. Interestingly, an Hsp90α-Aha 1 complex preferentially localized at the leading edge of migrating cancer cells, and KU-174 treatment shifted this localization to the cytosol and decreased the cell’s metastatic potential [57]. These are two examples of how disease-induced changes in the localization and composition of Hsp90 complexes can also contribute to the pharmacologic response to C-terminal Hsp90 inhibitors.
The above structure-activity relationship also affords an explanation for the neuroprotective properties of the second class of C-terminal Hsp90 modulators, KU-32 (Fig. 1c) [33, 52, 58, 59], and its fluorinated biphenyl derivative, the novologue KU-596 (Fig. 1d) [60, 61•]. The noviose sugar in KU-32 and KU-596 still allows binding to Hsp90α, but replacing the biaryl ring system present in KU-174 with an acetamide prevents Aha 1 dissociation and client protein degradation [57] (unpublished data). In contrast to KU-174, KU-32 and KU-596 improve mitochondrial bioenergetics, decrease oxidative stress, and aid neuronal survival [59, 62]. Interestingly, these cytoprotective responses were progressively lost by replacing the terminal methyl group of the acetamide tail on KU-32 with alkyl chains of increasing length. Whereas the presence of ethyl or propyl alkyl chains did not significantly increase cytoxicity relative to KU-32, lengthening the alkyl chain to 4–6 carbon atoms promoted dissociation of Aha 1, diminished the compound’s ability to activate the HSR, and increased the extent of cell death (unpublished data under review). This data clearly defines a structural point of divergence for interaction of the amide side chain with the C-terminal binding pocket that helps direct cytoprotective versus cytotoxic activities, presumably due to differentially altering the association of Aha 1 with Hsp90.
Lastly, although the noviose moiety is necessary for the interaction of KU-32, KU-174, and KU-596 with the C-terminal domain ofHsp90, molecular modeling suggested that it projects into a pocket within the C-terminal domain that may tolerate additional substitutions. Noviomimetics were designed as non-noviosylated analogs that extend into this pocket while retaining an ability to increase Hsp70 and avoid significant client protein degradation [63]. Assessment of noviomimetics containing furanose, cyclopentyl, or cyclohexyl rings identified that a cyclohexyl derivative with a 4-benzylether substitution (Fig. 1e) exhibited the best ability to activate an Hsp70 promoter and drive luciferase expression, indicative of promoting the HSR [63]. Based on this new scaffold, a lead noviomimetic has been identified, and its pharmacokinetic profile, potency, and efficacy in ameliorating symptoms of DPN compared to KU-596 are being evaluated.
In summary, the interaction of small molecules with Hsp90 heteroprotein complexes is influenced by many factors outside of in vitro affinity measurements of the drug for the chaperone alone [14]. Unfortunately, little is known about whether diabetes actually promotes the formation of stress-induced chaperome complexes. Similar to the efforts in oncology, determining whether diabetes may alter isoform-specific Hsp90 complexes and their interaction with small molecule modulators of Hsp90 will ultimately increase the potential potency while minimizing off-target effects of these agents. Despite this lack of molecular detail in what may regulate drug-Hsp90 interactions, modulating the diabetic chaperome is showing strong preclinical promise as a therapeutic option.
Targeting the Chaperome to Ameliorate Diabetes and Its Complications—How Might It Work and Is It Ready for Prime Time?
Numerous reports have generally shown that diabetes decreases the expression of Hsp70 and other members of the chaperome (reviewed in [18, 64, 65]). Though we have shown that increasing the expression of Hsp70 can improve DPN [3], neuroprotection is not limited to altering Hsp70. For example, mice overexpressing Hsp27 were resistant to developing a diabetic sensory neuropathy, and this correlated with a decrease in the extent of NF-κB activation [66]. Hsp27 may also be involved in human DPN.
In a 13-year longitudinal study in humans, Hsp27 significantly decreased from baseline in patients with type 1 diabetes and this correlated with the progression of a large fiber neuropathy [67]. Similarly, Hsp27 levels were also more decreased in type 2 diabetic patients versus those with normal or impaired glucose tolerance [68]. Though loss of function mutations in Hsp27 can cause an inherited human neuropathy [69], it is unlikely that a decrease in Hsp27 expression in diabetes promotes the neuropathy, but this has not been formally addressed.
Monitoring temporal changes in Hsp27 expression may provide a predictive biomarker for the severity of DPN. In a cross-sectional study of 119 diabetic patients, those with higher serum levels of Hsp27 showed better nerve function and fewer neuropathic symptoms than patients with lower levels of Hsp27 [68]. Although these data are consistent with a neuroprotective role of Hsp27, another cross-sectional study of 531 type 1 diabetic patients in the EURODIAB Prospective Complications Study found that higher serum Hsp27 levels correlated with the presence of DPN [70]. Unfortunately, the reasons for these discrepant findings remain unresolved. However, since an increase in Hsp27 protects against the development of DPN [66] and improves motor function [71], the observed increases in serum Hsp27 in humans likely reflect an endogenous neuroprotective response to the developing DPN. Since Hsp27 is a stress-inducible chaperone that can differentially interact with client proteins in stressed versus unstressed cells [72], its pharmacologic upregulation may minimize diabetic complications. However, pharmacologically modifying the expression of Hsp70 versus Hsp27 has been more successfully associated with attenuating insulin resistance as well as diabetic complications.
Pharmacologic approaches that modulate Hsp70 expression/activity are primarily indirect. BGP-15 (Fig. 1f) is a nicotinic amidoxime derivative which functions as a direct small molecule activator of HSF1 that improves skeletal muscle insulin resistance and overall glucose tolerance in an Hsp70-dependent manner [73, 74, 75•]. Short-term clinical trials found that BGP-15 administration (up to 400 mg/day) showed no adverse effects and improved insulin utilization in patients with impaired glucose tolerance [76] or insulin resistance consequent to olanzapine therapy [77]. Unfortunately, BGP-15 has not been tested in models of DPN, but it may directly improve the function of diabetic sensory neurons given its mechanism of action. In this regard, it is well recognized that mitochondrial dysfunction contributes to DPN [78, 79], and we have shown that modulating Hsp70 can improve this defect in diabetic sensory neurons [59]. Indeed, increasing Hsp70 expression with BGP-15 improves mitochondrial bioenergetics in skeletal muscle [73]. The drug may also aid the clearance of damaged mitochondria since Hsp70 is necessary for translocation of the E3 ubiquitin ligase, parkin, to depolarized mitochondria [75•]. Secondly, the activation of poly (ADP-ribose) polymerase (PARP1) has also been shown to be a key effector in the onset of DPN [80]. Interestingly, BGP-15 is not a specific activator of HSF1 since it also inhibits PARP1 [81]. Thus, BGP-15 is an Hsp70 modulator that seems well positioned to advance in treating insulin resistance or diabetic complications such as DPN. However, no results were reported of a terminated Phase II trial (NCT01069965) to assess its effectiveness as an add-on to metformin or metformin plus sulfonylurea therapy.
A second approach to indirectly increase Hsp70 is by targeting Hsp90 with N- or C-terminal modulators. AUY922 is a resorcinol derivative that targets the N-terminal of Hsp90 (Fig. 1g). Similar to BGP-15, AUY922 improved glucose regulation in the Leprdb/db mouse model of type 2 diabetes, and this correlated with an increase in Hsp70 expression [82]. Curiously, despite interacting at the same N-terminal site of Hsp90, the geldanamycin derivative DMAG (Fig. 1h) did not decrease blood glucose levels nor improve glycated hemoglobin levels (HbA1c). However, DMAG did improve diabetic nephropathy in a manner that likely required Hsp70 [19•, 20]. Nephroprotection by DMAG was associated with an inhibition of NF-κB and STAT activation leading to a decrease in inflammatory gene expression [19•]. Although both of these inhibitors bind the N-terminal nucleotide binding pocket, the ability of AUY-922 to improve glucose utilization suggests that it may more effectively modulate Hsp90 complexes in diabetic skeletal muscle, adipose, or liver compared to DMAG. It will be important to determine what underlies these distinct responses to a similar inhibitor class. If tissue-specific differences in the composition of diabetic Hsp90 complexes affect the drug-response phenotype, this can be exploited to selectively target diabetic complications without necessarily interfering with glucose regulation by established antidiabetic medications.
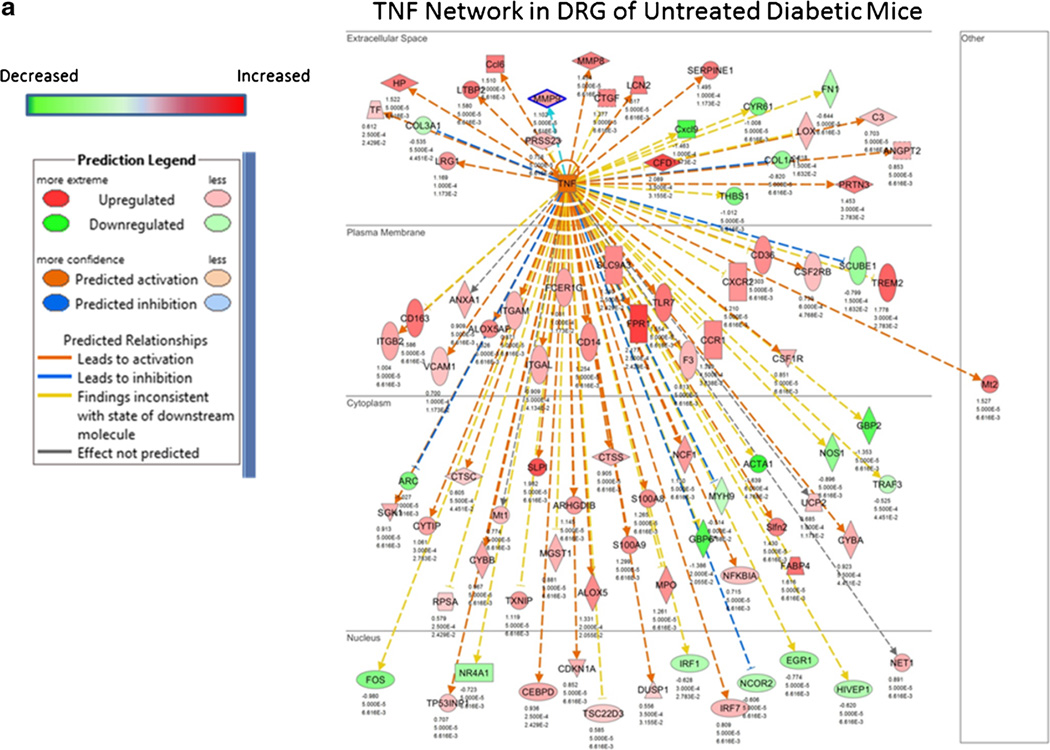
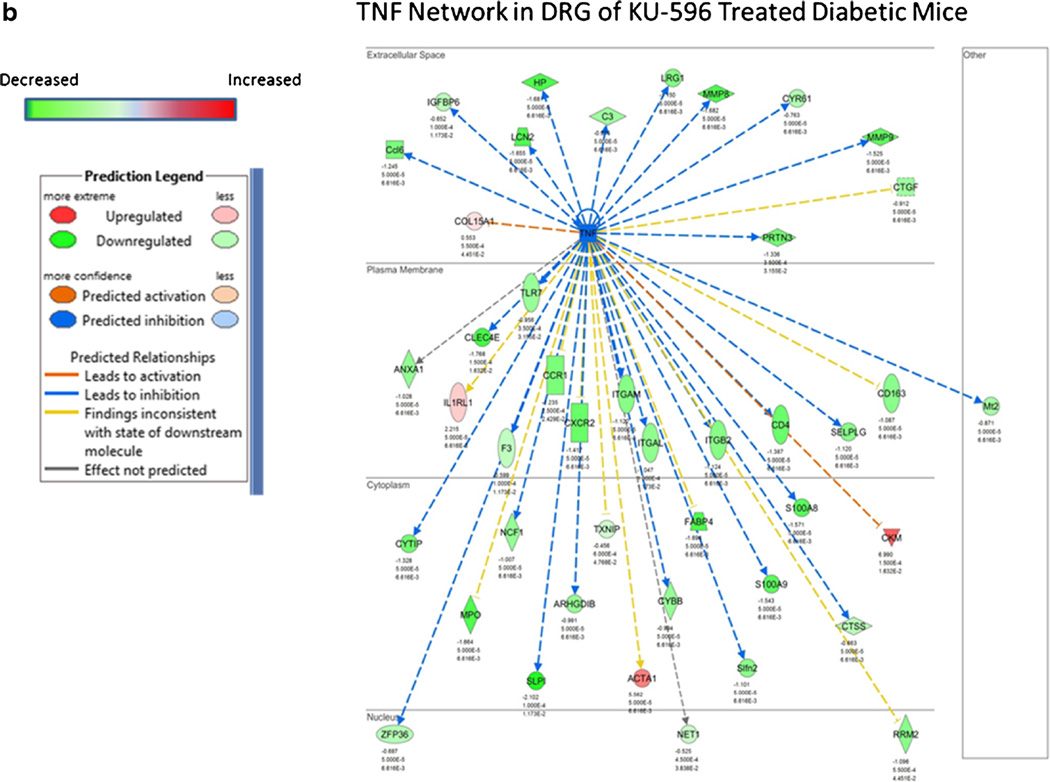
As previously mentioned, KU-32 and KU-596 are novobiocin derivatives that interact with the C-terminal dimerization domain of Hsp90. Both drugs are orally bio-available, rapidly distribute to tissue, and have a short half-life in serum and tissue [59]. Similar to the ability of DMAG to improve diabetic nephropathy, KU-32 and KU-596 improved preexisting psychosensory, electrophysiologic, morphologic, and bioenergetic deficits associated with the onset of DPN in type 1 and type 2 diabetic mouse models [58, 59, 61•, 83]. Comparable to DMAG, this neuroprotection also occurred without decreasing fasting blood glucose and HbA1c. Though KU-32 and KU-596 bind Hsp90 [54, 84], their ability to improve DPN is dependent on the presence of Hsp70 since the drugs were not effective in ameliorating symptoms of DPN in diabetic Hsp70 knockout (KO) mice [58, 59, 61•]. Mechanistically, recovery from the sensory neuropathy correlated with an Hsp70-dependent improvement in mitochondrial bioenergetics of the diabetic sensory neurons [59]. Though the underlying basis of how the chaperone improves mitochondrial function remains unclear, it may relate to a decrease in glucose-induced superoxide production in mitochondria [61•, 62], inhibition of various NADPH oxidase isoforms [85], or an Hsp70-dependent clearance of damaged organelles [72]. Similar to DMAG, KU-596 therapy also blocked a diabetes-induced increase in genes associated with inflammation [61•]. For example, the predicted activation state of genes associated with TNF signaling was generally increased in dorsal root ganglia from diabetic mice (Fig. 2a) but inhibited in diabetic mice receiving weekly therapy of 20 mg/kg KU-596 for 4 weeks (Fig. 2b) [61•]. Interestingly, KU-596 treatment also decreased the expression of genes associated with inflammation in diabetic Hsp70 KO mice [61•]. These data provide some of first evidence that this class of C-terminal Hsp90 modulator may affect DPN in both an Hsp70-dependent and independent manner. Whether this may be due to a differences in the interaction of the drug with Hsp90 complexes present in immune cells versus neurons is unknown.
Fig. 2.
Effect of diabetes and KU-596 therapy on the TNF network in dorsal root ganglion. C57Bl/6N mice were rendered diabetic using two injections of streptozotocin. After 12 weeks of diabetes, the mice were treated once per week for 4 weeks with 20 mg/kg of KU-596 or the drug vehicle, Captisol. Nondiabetic mice received similar treatments. After 16 weeks, the mice were euthanized and mRNA was isolated from the L4–L6 lumbar dorsal root ganglia (DRG) with three to five biologic replicates per group for RNA-Seq analysis. Differentially expressed genes were submitted to Ingenuity Pathway Analysis to identify and model the gene networks. The effect of diabetes on the predicted activation state of members of the TNF network in the absence (a) or presence (b) of KU-596 therapy is shown. Please see [61•] for additional experimental details
Conclusions
Although diabetic complications are not due to the formation of classic protein aggregates, diabetes can increase the accumulation of damaged proteins while impairing mechanisms for protein clearance and refolding [86]. The ability to modulate the chaperome to improve diabetic nephropathy and neuropathy provides proof-of-principle that it is appropriate to consider broadening our approach to invoke the potential benefits of targeting Hsp70 to treat diabetic complications. It would not be surprising that Hsp70 may improve diabetic complications via multiple mechanisms in different tissues via increasing proteasomal clearance of damaged proteins, chaperone-mediated autophagy, decreasing inflammation and oxidative stress, or improving mitochondrial function. However, Hsp70 overexpression is also a hallmark of certain malignancies [87]. Therefore, the magnitude and duration of modulating Hsp70 expression will be important to consider since long-term treatment would likely be necessary to ameliorate diabetic complications.
From a chemical biology perspective, whether Hsp90 modulators improve diabetic complications by drug interactions with “diabetic Hsp90 complexes”needs to be resolved. The emerging view in chaperone biology is that cellular stress leads to a mixture of Hsp90 chaperome complexes that serve distinct biologies. The housekeeping chaperome is essentially the contingent of species present in the nonstressed cell, while a disease-modified chaperome forms functionally distinct complexes that regulate the protein’s chaperone function, client protein activity, and localization [14]. For example, the immunophilin FK506-binding protein 51(FKBP51) is a co-chaperone that binds to the C-terminal domain of Hsp90. In response to noxious stimuli, FKBP51 increases in spinal cord dorsal horn neurons and its interaction with Hsp90 regulates the activity of the glucocorticoid receptor, an Hsp90 client, to promote nociception [88•]. Since inhibiting the prolyl isomerase activity of FKBP51 decreased mechanical hypersensitivity [88•], it will be of considerable interest to determine if C-terminal Hsp90 modulators may disrupt the interaction of FKBP51 with this pain-induced chaperone complex to also provide some protection against neuropathic pain.
In summary, much of what is known about the interaction of the various N- and C-terminal Hsp90 modulators with chaperone complexes comes from the extensive interest in developing these compounds as potential therapeutics for cancer. Although no Hsp90 modulator has been approved for clinical use, the efficacy of drugs such as DMAG, KU-32, and KU-596 in treating diabetic complications in animal models supports that targeting diabetes-induced alterations in proteostasis may afford a novel and effective disease-modifying approach for humans.
Acknowledgments
This review and the author’s work are supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [DK095911], the NIDDK Diabetes Complications Consortium (DiaComp) [DK076169], and the National Institute of Neurological Disorders and Stroke [NS075311].
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with humans and required no informed consent. Data generated with the use of animals were approved by the Institutional Animal Care and Use Committee and in compliance with standards and regulations for the care and use of laboratory rodents set by the National Institutes of Health.
Conflict of Interest The author declares a financial conflict through a license agreement with Reata Pharmaceuticals.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA. 2015;314:2172–2181. doi: 10.1001/jama.2015.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan BC, Cheng HT, Stables CL, et al. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer KL, Li C, Dobrowsky RT. Diabetic peripheral neuropathy: should a chaperone accompany our therapeutic approach? Pharmacol Rev. 2012;64:880–900. doi: 10.1124/pr.111.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Annals Intern Med. 2014;161:639–649. doi: 10.7326/M14-0511. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Alcala H, Santos Vichido CI, Islas Macedo S, et al. Treatment with alpha-lipoic acid over 16 weeks in type 2 diabetic patients with symptomatic polyneuropathy who responded to initial 4-week high-dose loading. J Diab Res. 2015:189857. doi: 10.1155/2015/189857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the Nathan 1 Trial. Diab Care. 2011;34:2054–2060. doi: 10.2337/dc11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler D, Low PA, Freeman R, et al. Predictors of improvement and progression of diabetic polyneuropathy following treatment with alpha-lipoic acid for 4 years in the Nathan 1 Trial. J Diab Complic. 2016;2:350–356. doi: 10.1016/j.jdiacomp.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Calcutt NA, Cooper ME, Kern TS, et al. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–430. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calcutt NA. Tolerating diabetes - an alternative therapeutic approach for diabetic neuropathy. ASN Neuro. 2010;2:215–217. doi: 10.1042/AN20100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 11.Chittoor-Vinod VG, Lee S, Judge SM, et al. Inducible Hsp70 is critical in preventing the aggregation and enhancing the processing ofPMP22. ASN Neuro. 2015;7 doi: 10.1177/1759091415569909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chittoor VG, Sooyeon L, Rangaraju S, et al. Biochemical characterization of protein quality control mechanisms during disease progression in the C22 mouse model of CMT1A. ASN Neuro. 2013;5:e00128. doi: 10.1042/AN20130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Venable J, LaPointe P, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Taldone T, Ochiana SO, Patel, et al. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol Sci. 2014;35:592–603. doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finka A, Goloubinoff P. Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chap. 2013;18:591–605. doi: 10.1007/s12192-013-0413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson LB, Blagg BS. To fold or not to fold: modulation and consequences of Hsp90 inhibition. Future Med Chem. 2009;1:267–283. doi: 10.4155/fmc.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and Type 2 diabetes. Mol Metab. 2014;3:781–793. doi: 10.1016/j.molmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazaro I, Oguiza A, Recio C, et al. Targeting Hsp90 ameliorates nephropathy and atherosclerosis through suppression of NF-kappaB and STAT signaling pathways in diabetic mice. Diabetes. 2015;64:3600–3613. doi: 10.2337/db14-1926. A clear characterization of an N-terminal Hsp90 inhibitor decreasing measures of diabetic nephropathy via inhibition of inflammatory signaling in a potentially Hsp70-dependent manner.
- 20.Zhang HM, Dang H, Kamat A, et al. Geldanamycin derivative ameliorates high fat diet-induced renal failure in diabetes. PLoS One. 2012;7:e32746. doi: 10.1371/journal.pone.0032746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulick K, Ahn JH, Zong H, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nature Chem Biol. 2011;7:818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finka A, Mattoo RU, Goloubinoff P. Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chap. 2011;16:15–31. doi: 10.1007/s12192-010-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brocchieri L, Conway de Macario E, Macario AJ. Hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radons J. The human Hsp70 family of chaperones: where do we stand? Cell Stress Chap. 2016 doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker SL, Kampinga HH, Bergink S. DnaJs: more than substrate delivery to HspA. Frontiers Mol Biosci. 2015;2:35. doi: 10.3389/fmolb.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assimon VA, Gillies AT, Rauch JN, et al. Hsp70 protein complexes as drug targets. Curr Pharmaceut Design. 2013;19:404–417. doi: 10.2174/138161213804143699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vihervaara A, Sistonen L. HSF1 at a glance. J Cell Sci. 2014;127:261–266. doi: 10.1242/jcs.132605. [DOI] [PubMed] [Google Scholar]
- 28.Neef DW, Jaeger AM, Thiele DJ. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov. 2011;10:930–944. doi: 10.1038/nrd3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangaraju S, Madorsky I, Pileggi JG, et al. Pharmacological induction of the heat shock response improves myelination in a neuropathic model. Neurobiol Dis. 2008;32:105–115. doi: 10.1016/j.nbd.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jinwal UK, Akoury E, Abisambra JF, et al. Imbalance of hsp70 family variants fosters tau accumulation. FASEB J. 2013;27:1450–1459. doi: 10.1096/fj.12-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koren J, Jinwal UK, Jin Y, et al. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J Biol Chem. 2010;285:2498–2505. doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo W, Dou F, Rodina A, et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci. 2007;104:9511–9516. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Ma J, Zhao H, et al. Induction of heat shock protein 70 (Hsp70) prevents neuregulin-induced demyelination by enhancing the proteasomal clearance of c-jun. ASN Neuro. 2012;4:425–437. doi: 10.1042/20120047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehi AH, Morris SJ, Ho WC, et al. AEG3482 is an antiapoptotic compound that inhibits jun kinase activity and cell death through induced expression of heat shock protein 70. Chem Biol. 2006;13:213–223. doi: 10.1016/j.chembiol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Waza M, Adachi H, Katsuno M, et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- 36.Ciglia E, Vergin J, Reimann S, et al. Resolving hot spots in the C-terminal dimerization domain that determine the stability of the molecular chaperone Hsp90. PLoS One. 2014;9:e96031. doi: 10.1371/journal.pone.0096031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall JA, Forsberg LK, Blagg BS. Alternative approaches to Hsp90 modulation for the treatment of cancer. Future Med Chem. 2014;6:1587–1605. doi: 10.4155/fmc.14.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clerico EM, Tilitsky JM, Meng How hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J Mol Biol. 2015;427:1575–1588. doi: 10.1016/j.jmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharmaceut Design. 2013;19:347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 41.Beebe K, Mollapour M, Scroggins B, Prodromou C, Xu W, Tokita M, et al. Posttranslational modification and conformational state of heat shock protein 90 differentially affect binding of chemically diverse small molecule inhibitors. Oncotarget. 2013;4:1065–1074. doi: 10.18632/oncotarget.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mollapour M, Bourboulia D, Beebe K, et al. Asymmetric Hsp90 N-domain SUMOylation recruits Aha1 and ATP-competitive inhibitors. Mol Cell. 2014;53:317–329. doi: 10.1016/j.molcel.2013.12.007. An excellent molecular characterization of how post-translational modification of Hsp90 can sensitize cells to N-terminal Hsp90 inhibitors.
- 43.Prince TL, Kijima T, Tatokoro, et al. Client proteins and small molecule inhibitors display distinct binding preferences for constitutive and stress-induced Hsp90 isoforms and their conformationally restricted mutants. PloS Oone. 2015;10:e0141786. doi: 10.1371/journal.pone.0141786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carman A, Kishinevsky S, Koren J, 3rd, et al. Chaperone-dependent neurodegeneration: a molecular perspective on therapeutic intervention. J Alz Dis Parkinson. 2013;10:7. doi: 10.4172/2161-0460.S10-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujikake N, Nagai Y, Popiel HA, et al. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem. 2008;283:26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dou F, Netzer WJ, Tanemura K, et al. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eskew JD, Sadikot T, Morales P, et al. Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer. 2011;11:468. doi: 10.1186/1471-2407-11-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Vielhauer GA, Holzbeierlein JM, et al. KU675, a concomitant heat-shock protein inhibitor of Hsp90 and Hsc70 that manifests isoform selectivity for Hsp90alpha in prostate cancer cells. Mol Pharmacol. 2015;88:121–130. doi: 10.1124/mol.114.097303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samadi AK, Zhang X, Mukerji R, et al. A novel C-terminal Hsp90 inhibitor KU135 induces apoptosis and cell cycle arrest in melanoma cells. Cancer Lttrs. 2011;312:158–167. doi: 10.1016/j.canlet.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao H, Anyika M, Girgis A, et al. Novologues containing a benzamide side chain manifest anti-proliferative activity against two breast cancer cell lines. Bioorg Med Chem Lttrs. 2014;24:3633–3637. doi: 10.1016/j.bmcl.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansar S, Burlison JA, Hadden MK, et al. A non-toxic Hsp90 inhibitor protects neurons from Abeta-induced toxicity. Bioorg Med Chem Lttrs. 2007;17:1984–1990. doi: 10.1016/j.bmcl.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 53.Lu Y, Ansar S, Michaelis ML, et al. Neuroprotective activity and evaluation of Hsp90 inhibitors in an immortalized neuronal cell line. Bioorg Med Chem. 2008;17:1709–1715. doi: 10.1016/j.bmc.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matts RL, Dixit A, Peterson LB, et al. Elucidation of the Hsp90 C-terminal inhibitor binding site. ACS Chem Biol. 2011;6:800–807. doi: 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratzke C, Mickler M, Hellenkamp B, et al. Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle. Proc Natl Acad Sci Am. 2010;107:16101–16106. doi: 10.1073/pnas.1000916107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Retzlaff M, Stahl M, Eberl HC, et al. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Repts. 2009;10:1147–1153. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh S, Shinogle HE, Garg G, et al. Hsp90 c-terminal inhibitors exhibit antimigratory activity by disrupting the Hsp90alpha/Aha1 complex in PC3-MM2 cells. ACS Chem Biol. 2015;10:577–590. doi: 10.1021/cb5008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urban MJ, Li C, Yu C, et al. Inhibiting heat shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro. 2010;2:189–199. doi: 10.1042/AN20100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma J, Farmer KL, Pan P, et al. Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J Pharmacol Exp Therapeu. 2014;348:281–292. doi: 10.1124/jpet.113.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kusuma BR, Zhang L, Sundstrom T, et al. Synthesis and evaluation of novologues as C-terminal Hsp90 inhibitors with cytoprotective activity against sensory neuron glucotoxicity. J Med Chem. 2012;55:5797–5812. doi: 10.1021/jm300544c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ma J, Pan P, Anyika M, et al. Modulating molecular chaperones improves mitochondrial bioenergetics and decreases the inflammatory transcriptome in diabetic sensory neurons. ACS Chem Neurosci. 2015;6:1637–1648. doi: 10.1021/acschemneuro.5b00165. First report of Hsp70-dependent and Hsp70-independent effects of a C-terminal Hsp90 modulator in improving diabetic peripheral neuropathy.
- 62.Zhang L, Zhao H, Blagg BS, et al. A C-terminal heat shock protein 90 inhibitor decreases hyperglycemia-induced oxidative stress and improves mitochondrial bioenergetics in sensory neurons. J Proteome Res. 2012;11:129–137. doi: 10.1021/pr300056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anyika M, McMullen M, Forsberg LK, et al. Development of noviomimetics as C-terminal Hsp90 inhibitors. ACS Med Chem Lttrs. 2015;7:67–71. doi: 10.1021/acsmedchemlett.5b00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atalay M, Oksala N, Lappalainen J, et al. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10:85–95. doi: 10.2174/138920309787315202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padmalayam I. The heat shock response: its role in pathogenesis of Type 2 diabetes and its complications, and implications for therapeutic intervention. Discov Med. 2014;18:29–39. [PubMed] [Google Scholar]
- 66.Korngut L, Ma CH, Martinez JA, et al. Overexpression of human Hsp27 protects sensory neurons from diabetes. Neurobiol Dis. 2012;47:436–443. doi: 10.1016/j.nbd.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pourhamidi K, Skarstrand H, Dahlin LB, et al. Hsp27 concentrations are lower in patients with Type 1 diabetes and correlate with large nerve fiber dysfunction. Diab Care. 2014;37:e49–e50. doi: 10.2337/dc13-1780. [DOI] [PubMed] [Google Scholar]
- 68.Pourhamidi K, Dahlin LB, Boman K, et al. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia. 2011;54:3143–3149. doi: 10.1007/s00125-011-2303-5. [DOI] [PubMed] [Google Scholar]
- 69.Pareyson D, Saveri P, Piscosquito G. Charcot-marie-tooth disease and related hereditary neuropathies: from gene function to associated phenotypes. Current Mol Med. 2014;14:1009–1033. doi: 10.2174/1566524014666141010154205. [DOI] [PubMed] [Google Scholar]
- 70.Gruden G, Bruno G, Chaturvedi N, et al. Serum heat shock protein 27 and diabetes complications in the Eurodiab Prospective Complications Study: a novel circulating marker for diabetic neuropathy. Diabetes. 2008;57:1966–1970. doi: 10.2337/db08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma CH, Omura T, Cobos EJ, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibert B, Eckel B, Fasquelle L, et al. Knock down of heat shock protein 27 (Hspb1) induces degradation of several putative client proteins. PLoS One. 2012;7:e29719. doi: 10.1371/journal.pone.0029719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henstridge DC, Bruce CR, Drew BG, et al. Activating hsp72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung J, Nguyen AK, Henstridge DC, et al. Hsp72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Drew BG, Ribas V, Le JA, et al. Hsp72 is a mitochondrial stress sensor critical for parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 2014;63:1488–1505. doi: 10.2337/db13-0665. A compelling study that provides molecular insight into how Hsp70 may improve mitochondrial quality and function.
- 76.Literáti-Nagy B, Kulcsár E, Literáti-Nagy Z, et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41:374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- 77.Literati-Nagy B, Peterfai E, Kulcsar E, et al. Beneficial effect of the insulin sensitizer (Hsp inducer) BGP-15 on olanzapine-induced metabolic disorders. Brain Res Bull. 2010;83:340–344. doi: 10.1016/j.brainresbull.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Chowdhury SK, Smith DR, Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56–65. doi: 10.1016/j.nbd.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Chowdhury SKR, Smith DR, Saleh A, et al. Impaired AMP-activated protein kinase signaling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135:1751–1766. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Obrosova IG, Xu W, Lyzogubov VV, et al. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Rad Biol Med. 2008;44:972–981. doi: 10.1016/j.freeradbiomed.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szabados E, Literati-Nagy P, Farkas B, et al. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(adp-ribose) polymerase. Biochem Pharmacol. 2000;59:937–945. doi: 10.1016/s0006-2952(99)00418-9. [DOI] [PubMed] [Google Scholar]
- 82.Lee JH, Gao J, Kosinski PA, et al. Heat shock protein 90 (Hsp90) inhibitors activate the heat shock factor 1 (Hsf1) stress response pathway and improve glucose regulation in diabetic mice. Biochem Biophys Res Commun. 2013;430:1109–1113. doi: 10.1016/j.bbrc.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 83.Urban MJ, Pan P, Farmer KL, et al. Modulating molecular chaperones improves sensory fiber recovery and mitochondrial function in diabetic peripheral neuropathy. Exp Neurol. 2012;235:388–396. doi: 10.1016/j.expneurol.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matts RL, Brandt GE, Lu Y, et al. A systematic protocol for the characterization of Hsp90modulators. Bioorg Med Chem. 2011;19:684–692. doi: 10.1016/j.bmc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen F, Haigh S, Yu Y, et al. Nox5 stability and superoxide production is regulated by C-terminal binding of Hsp90 and co-chaperones. Free Rad Biol Med. 2015;89:793–805. doi: 10.1016/j.freeradbiomed.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaisson S, Gillery P. Impaired proteostasis: role in the pathogenesis of diabetes mellitus. Diabetologia. 2014;57:1517–1527. doi: 10.1007/s00125-014-3257-1. [DOI] [PubMed] [Google Scholar]
- 87.Lianos GD, Alexiou GA, Mangano A, et al. The role of heat shock proteins in cancer. Cancer Lttrs. 2015;360:114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 88. Maiaru M, Tochiki KK, Cox MB, et al. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Sci Transl Med. 2016;8:325ra19. doi: 10.1126/scitranslmed.aab3376. A very convincing demonstration of how stress-induced changes in an Hsp90-immunophilin complex can contribute to the development of a painful neuropathy.