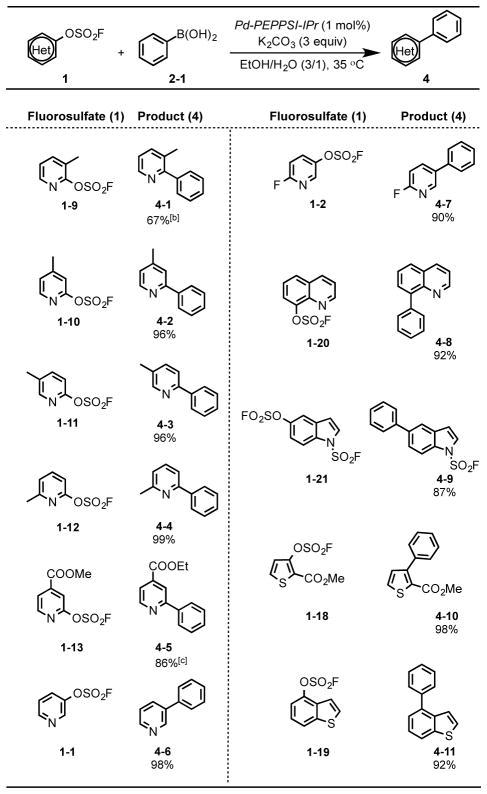
Table 3.
Suzuki reactions of heteroaryl fluorosulfates (1) with phenylboronic acid 2-1.[a]
Reaction conditions: 1 (1.0 mmol), phenylboronic acid 2-1 (1.5 mmol), Pd-PEPPSI-IPr (1 mol%), K2CO3 (3.0 mmol), EtOH/H2O (10 mL, 3/1), in nitrogen at 35 °C.
The reaction was run with 2 mol% of Pd-PEPPSI-IPr.
Transesterification to the ethyl ester occurred under the reaction conditions.