Figure 1.
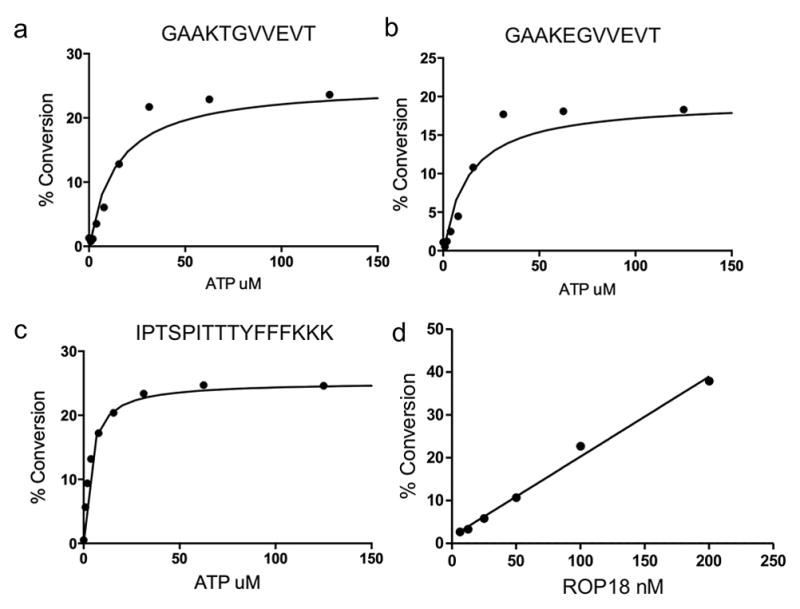
Kinetic analysis of ROP18 kinase. Determination of the apparent ATP Km for three fluorescently labeled ROP18 substrates in their respective microfluidic assays: (a) FL-T, a native ROP18 substrate peptide from Irga6 (b) FL-E, a threonine point mutation of the native peptide from Irga6, and (c) FL-8, an unrelated peptide substrate. Percent substrate conversion to phosphorylated product was plotted against ATP concentration and fit to the Michaelis-Menton equation to determine the ATP apparent Km for each of the three substrates. All titrations contained 1 μM peptide; a and b were conducted with 50 nM ROP18, while c was conducted with ~15 nM ROP18. (d) Determination of optimal enzyme concentration for assay. Recombinant ROP18 kinase was titrated using FL-T substrate to determine the amount of enzyme needed to obtain ~30% conversion to phosphorylated product after a 3 h room temperature incubation.
