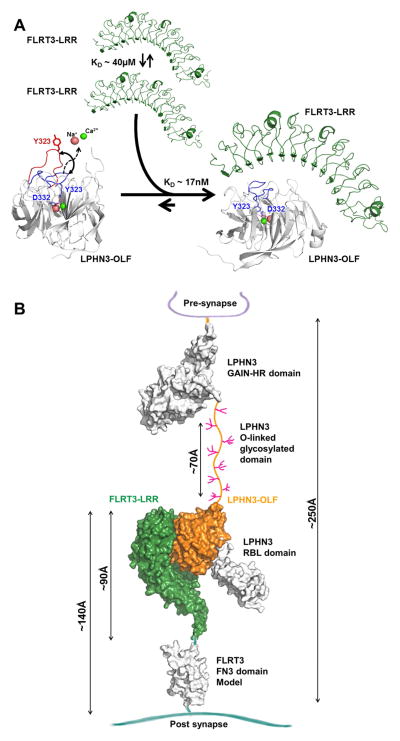
Figure 6. Overall model of the association in the context of the synapse.
(A), FLRT-LRR alone tends to weakly dimerize and LPHN3-OLF alone has loop 316-329 that is mobile possibly allowing and Ca2+ and Na+ exchange. The high affinity of the association maintains LPHN3-OLF loop 316–329 closed and dissociates the low-affinity homo-dimerization of FLRT3-LRR. (B), LPHN3 is presynaptic and it binds through the OLF domain to the LRR domain of FLRT3, a post-synaptic protein. With the exception of the central O-linked glycosylated stalk domain drawn between the GAIN domain and the OLF domain, the rest of extracellular domain of LPHN3 has been determined using crystallography. FLRT3 FN3 domain is represented as homology model using other FN3 domains as template. Approximate dimensions are shown in Angstrom (Å).