Abstract
Consumption of high doses of ethanol can lead to amnesia, which often manifests as a blackout. These blackouts experienced by ethanol consumers may be a major cause of the social problems associated with excess ethanol consumption. However, there is currently no established treatment for preventing these ethanol-induced blackouts. In this study, we tested the ethanol extract of the roots of Salvia miltiorrhiza (SM) for its ability to mitigate ethanol-induced behavioral and synaptic deficits. To test behavioral deficits, an object recognition test was conducted in mouse. In this test, ethanol (1 g/kg, i.p.) impaired object recognition memory, but SM (200 mg/kg) prevented this impairment. To evaluate synaptic deficits, NMDA receptor-mediated excitatory postsynaptic potential (EPSP) and long-term potentiation (LTP) in the mouse hippocampal slices were tested, as they are known to be vulnerable to ethanol and are associated with ethanol-induced amnesia. SM (10 and 100 μg/ml) significantly ameliorated ethanol-induced long-term potentiation and NMDA receptor-mediated EPSP deficits in the hippocampal slices. Therefore, these results suggest that SM prevents ethanol-induced amnesia by protecting the hippocampus from NMDA receptor-mediated synaptic transmission and synaptic plasticity deficits induced by ethanol.
Keywords: Salvia miltiorrhiza, Ethanol, Synaptic plasticity, NMDA receptor
INTRODUCTION
Ethanol is a widely consumed recreational substance. While low doses of ethanol are believed to be beneficial for brain function, high doses of ethanol can cause detrimental effects including memory impairment, which may manifest as a blackout. The ensuing blackouts are known to be related to the function of the hippocampus, and can lead to various social problems. Although caffeine has been reported to reduce the various detrimental effects of ethanol, including sedative effect, amnesia, and loss of motor coordination (Liguori and Robinson, 2001; Gulick and Gould, 2009; Spinetta et al., 2008), there is still no treatment for preventing this ethanol-induced neurological impairment.
Synaptic plasticity is believed to be involved in various functions of the brain, and NMDA receptor-dependent long-term potentiation (LTP) has long been studied as a cellular model of memory processing (Jaffe and Johnston, 1990; Komatsu et al., 1991; Johnston et al., 1992; Castillo et al., 1994). Ethanol induces inhibition of excitatory glutamate ion channel receptors (Lovinger et al., 1989; He et al., 2013) and also potentiates inhibitory GABA transmission in the central nervous system (Aguayo, 1990; Allan and Harris, 1987). Together, these two processes result in synaptic dysfunction (Chandler, 2003). The GABAergic system contributes to the function of synaptic NMDARs (Steffensen et al., 2000), suggesting that GABAergic modulation may regulate the effect of ethanol on NMDA receptor and synaptic plasticity.
Salvia miltiorrhiza Bunge (Labiatae; SM) is a widely used natural product for treating a wide range of cardiovascular conditions (Cheng, 2007). Recent studies reported that the root extract of SM protected against ethanol-induced liver damage and encouraged the consumer to drink less alcohol (Lu et al., 2012; Li et al., 2014). Moreover, we recently reported that some of constituents of SM are antagonists of the GABAA receptor (Kim et al., 2007). Therefore, we hypothesized that the GABAergic effect of SM may regulate ethanol-induced changes in NMDA receptor function, and the function of the CNS as a whole. However, the direct neurological effect of SM on ethanol-induced behavioral changes is yet to be studied. To prove this hypothesis, we tested whether SM is able to regulate NMDA receptor function and synaptic plasticity. In addition, we studied the effect of SM on ethanol-induced memory impairment and uncontrolled body movement.
MATERIALS AND METHODS
Materials
Ethanol was purchased from Sigma-Aldrich (St. Louis, MO, USA). NBQX was purchased from Tocris Bioscience (Ellisville, MO, USA). All of the other materials were of the highest grade available and were obtained from normal commercial sources. The roots of Salvia miltiorrhiza BUNGE (Labiatae) were purchased from the Kyungdong oriental drug store (Seoul, Korea). The roots of Salvia miltiorrhiza were extracted with 70% ethanol three times for 2 h under an ultrasonic apparatus. The ethanolic solution obtained was filtered, concentrated in a water bath under vacuum, frozen, and lyophilized (model FD-5N; Eyela, Tokyo, Japan) to yield the 70% ethanolic extract (defined as SM), which was then stored at −20°C until required (yield: 8.5%). SM was standardized based on the contents of tanshinone IIA (0.0595%). The chromatographic system consisted of a LC-10AT pump, a SIL-10AF autosampler and a SPD-10Avp UV-VIS detector (Shimadzu, Kyoto, Japan). A Purospher® STAR RP-18 endcapped (250 mm×4.6 mm i.d., 5 μm) (Merck, Darmstadt, Germany) was applied for the separation and temperature was controlled at 25°C. The mobile phase was made up of 75% acetonitrile, 12.5% methanol and 12.5% water at volumetric ratios and pH was titrated to 3.0 with glacial acetic acid. The detection was monitored at 270 nm. The mobile phase was delivered at a rate of 1.0 mL/min and the volume of injection loop was 10 μL. Diazepam was used as the internal standard. The sample calibration curve for tanshinone IIA was linear (r=0.9999) within the range 0–50 mg/mL. Intra- and inter-day coefficients of variation of the assays were less than 5% (n=6).
Animals
Male CD-1 mice (8 weeks) were purchased from SAMTA-KO biokorea (Osan, Korea), and kept in the University Animal Care Unit for 1 week prior to the experiments. The animals were housed 5 per cage, allowed access to water and food ad libitum; the environment was maintained at a constant temperature (23 ± 1°C) and humidity (60 ± 10%) under a 12-h light/dark cycle (the lights were on from 07:30 to 19:30). The treatment and maintenance of the animals were carried out in accordance with the Animal Care and Use Guidelines of Dong-A University, Busan, Korea. All of the experimental protocols using animals approved by the Institutional Animal Care and Use Committee of Dong-A University, Busan, Korea.
Slice preparation and extracellular recording
Mouse hippocampal slices were prepared using micro-vibratome (Lafayette-campden neuroscienceTM). The brain was rapidly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF; bubbled with 95% O2/5% CO2), which comprised: (mM) NaCl, 124; KCl, 3; NaHCO3, 26; NaH2PO4, 1.25; CaCl2, 2; MgSO4, 1; D-glucose, 10. Transverse hippocampal slices (400 μm thick) were prepared. Hippocampal slices were submerged in ACSF (20°–25°C) for 1 h before transfer to the recording chamber (28°–30°C, flow rate ∼3 ml/min) as required.
Field recordings were made from stratum pyramidale in area CA1. Stimulating electrodes were placed in the Schaffer collateral-commissural pathway. Stimuli (constant voltage) were delivered at 30 s intervals. To isolate NMDAR-mediated field excitatory postsynaptic potential (fEPSP), NBQX (50 μM) was perfused during recording. To induce LTP, one train of high frequency stimulation (HFS, 100 pulses at 100 Hz) was delivered. The slope of the evoked field potential responses were averaged from four consecutive recordings (EPSPs) evoked at 30 s intervals. Ethanol (80 mM) was used in electro-physiology experiments.
Object recognition test
The experimental apparatus consisted of a black rectangular open field (25 cm×25 cm×25 cm). The object recognition task was carried out as described elsewhere (Yi et al., 2016). Habituation training took place by exposing the animal to the experimental apparatus for 10 min in the absence of objects. Sample sessions were conducted 30 min after Ethanol (1 g/kg) injection. During the sample sessions, mice were placed in the experimental apparatus in the presence of two identical objects and allowed to explore for 10 min. After a retention interval of 24 h, mice were placed again in the apparatus, where a novel one replaced one of the objects. Mice were allowed to explore for 5 min. The durations of time mice spent exploring each object (familiar object, Tfamiliar; novel object, Tnovel) were recorded. The discrimination ratio was calculated by following formula: (Tnovel−Tfamiliar)/(Tnovel+Tfamiliar). The objects were a metal cylinder and plastic rectangular block with approximately the same height. SM was administered 30 min before Ethanol injection.
Statistics
Data were analyzed by one-way analysis of variance (ANO-VA) followed by Tukey’s test for multiple comparisons. Statistical significance was determined at p<0.05.
RESULTS
SM rescues binge ethanol-induced memory impairment
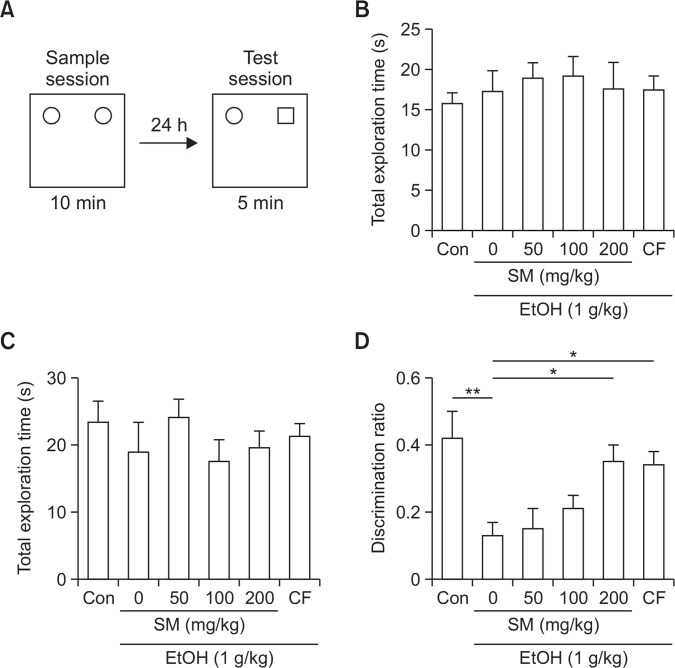
To test the effect of SM on ethanol-induced memory impairment, we performed an object recognition test. SM was administered 30 min prior to the ethanol administration. Ethanol (1 g/kg, i.p.) was administered 30 min prior to the training session. Test session was conducted 24 h later (Fig. 1A). Acute administration of ethanol (1 g/kg) significantly impaired recognition memory and SM protected against ethanol-induced memory impairment (Fig. 1D) without affecting the total exploration time (Fig. 1B, 1C).
Fig. 1.
SM rescues ethanol-induced recognition impairment. (A) SM was orally treated 30 min before Ethanol treatment. Ethanol was intra-peritoneally injected 30 min before sample session. Test session was conducted 24 h after the sample session. (B) Total exploration time during sample test. (C) Total exploration time during test session. (D) Discrimination ratio during test session. Data represent mean ± SEM. *p<0.05. **p<0.01. EtOH, ethanol. SM, Salvia miltiorrhiza extract. CF, caffeine.
SM ameliorates ethanol-induced NMDAR-mediated fEPSP impairment
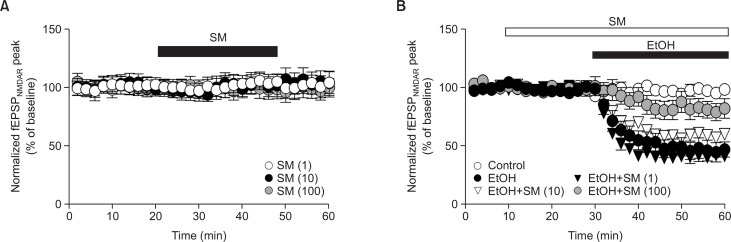
Previous reports have suggested that ethanol impairs hippocampal NMDAR-mediated fEPSP (fEPSPNMDAR), and this may be a mechanism of ethanol-induced memory impairment (Hicklin et al., 2011). Therefore, we tested the effect of SM on ethanol-induced NMDAR fEPSP (fEPSPNMDAR) impairment in the hippocampus. To isolate fEPSPNMDAR, we perfused NBQX (50 μM) (Alvestad et al., 2003), an AMPAR antagonist. SM itself did not affect fEPSPNMDAR (Fig. 2A). Ethanol (80 mM) reduced fEPSPNMDAR, but SM blocked this in a concentration-dependent manner (Fig. 2B).
Fig. 2.
SM rescues ethanol-induced decrease of fEPSPNMDAR. To isolate fEPSPNMDAR, NBQX (50 μM) was perfused during recording. The peak of the evoked field potential responses were averaged from four consecutive recordings (EPSPs) evoked at 30 s intervals (Ampk). (A) The effect of SM on fEPSPNMDAR. (B) SM reduced ethanol-induced decrease of fEPSPNMDAR in a concentration dependent manner. Data were normalized to baseline (0–20 min) and represent mean ± S.E.M. EtOH, ethanol. SM, Salvia miltiorrhiza extract.
SM blocks ethanol-induced LTP impairment
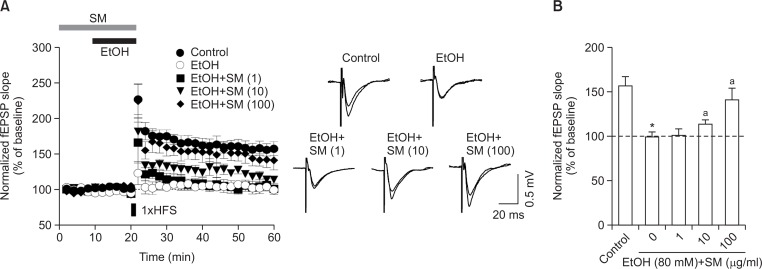
To test the effect of SM on ethanol-induced memory impairment, we next examined LTP, which has been previously reported to be impaired by ethanol (Hicklin et al., 2011; Izumi et al., 2005). Ethanol (80 mM) reduced LTP (Control, 174 ± 8; ethanol, 109 ± 6%, Fig. 3A), while SM blocked this ethanol-induced reduction in LTP in a concentration-dependent manner (1 μg/ml, 123 ± 9%, n=6; 10 μg/ml, 138 ± 5%, n=6; 100 μg/ml, 158 ± 11%, n=6, Fig. 3B).
Fig. 3.
SM rescues ethanol-induced LTP impairment. (A) SM was perfused from 20 min before ethanol perfusion. To induce LTP, one train of high frequency stimulation (HFS, 100 pulses at 100 Hz) was delivered. (B) Quantitative analysis of fEPSP slope at 40 min after HFS treatment. Data were normalized to baseline (0–20 min) and represent mean ± S.E.M. *p<0.05 vs. control group. ap<0.05 vs EtOH only group. EtOH, ethanol. SM, Salvia miltiorrhiza extract.
DISCUSSION
High consumption of ethanol can lead to social problems, which demand a significant investment of public funds and time to resolve. There is still no solution, pharmacological or otherwise, to these problems. The biggest neurological problems of ethanol intoxication is amnesia. In the present study, we found that SM reduced the impact of ethanol-induced amnesia by reducing the effect of ethanol-induced loss of synaptic plasticity and ethanol-induced loss of NMDA receptor-mediated synaptic transmission. As a result, SM is an excellent candidate for further development towards the individual pharmacological treatment of ethanol-induced neurological damage.
There is evidence that ethanol blocks NMDAR-dependent forms of LTP in the hippocampus (Chandler, 2003). The NMDAR-dependent form of LTP in the hippocampus has been proposed as a crucial mechanism of learning and memory (Jaffe and Johnston, 1990; Johnston et al., 1992; Komatsu et al., 1991; Castillo et al., 1994). NMDAR antagonists block NMDAR-dependent LTP and learning. It then follows that the function of NMDARs is important for LTP induction in the hippocampus. Ethanol is known to inhibit NMDARs (Dodd et al., 2000; Allgaier, 2002). Some studies have suggested that only some specific subtypes of NMDARs are affected by ethanol and are subsequently culpable for ethanol-induced deficits to memory and cognition (Dodd et al., 2000; Yaka et al., 2003; Ron, 2004). The cellular mechanism suggested is that ethanol-induced reduction of NMDAR function is mediated by dephosphorylation of tyrosine residues on specific subunits of NMDARs including NR2A and NR2B (Alvestad et al., 2003); tyrosine phosphatase is suspected as the causative agent for this phenomenon (Hicklin et al., 2011). Moreover, broad-spectrum inhibitors of tyrosine phosphatase diminished ethanol-induced reduction of NMDAR-dependent fEPSP. Previous reports have found that abietane diterpenes isolated from SM have an inhibitory effect on tyrosine phosphatase 1B (Han et al., 2005). In the present study, SM reduced ethanol-induced reduction of NMDAR-dependent fEPSP in the hippocampus. This suggests that SM may block ethanol-induced tyrosine phosphatase activation, reducing ethanol’s ability to affect NMDAR-dependent fEPSP.
In addition, previous studies have indicated that ethanol facilitates GABAergic inhibition (Hanchar et al., 2004; Koob, 2004). The GABAergic system contributes to effects on synaptic NMDARs (Steffensen et al., 2000). Izumi et al. (2005) reported that the effects of 60 mM ethanol on LTP are overcome by picrotoxin, a GABAA receptor antagonist. Additionally, low concentrations of ethanol augment tonic GABAergic inhibition (Wallner et al., 2003; Wei et al., 2004) and that tonic GABA currents in CA1 interneurons are sensitive to being blocked by low concentrations of picrotoxin (Semyanov et al., 2003). This indicates that the effects of ethanol on GABAA receptor-mediated inhibition contribute to the reduction of LTP in the CA1 region. Previously, we reported that tanshinone congeners isolated from SM demonstrated a protective effect on cholinergic dysfunction-induced memory impairment through its GABAergic inhibitory action (Kim et al., 2007). This suggests that the GABAergic inhibitory action of SM may also explain the action of SM to reduce ethanol-induced LTP impairment.
Acknowledgments
This research was supported by Dong-A University research supporting program.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests.
REFERENCES
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl-current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-B. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/S0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-D-aspartate receptor function. J Biol Chem. 2003;278:11020–11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/S0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/S0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Effects of ethanol and caffeine on behavior in C57BL/6 mice in the plus-maze discriminative avoidance task. Behav Neurosci. 2009;123:1271–1278. doi: 10.1037/a0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YM, Oh H, Na M, Kim BS, Oh WK, Kim BY, Jeong DG, Ryu SE, Sok DE, Ahn JS. PTP1B inhibitory effect of abietane diterpenes isolated from Salvia miltiorrhiza. Biol Pharm Bull. 2005;28:1795–1797. doi: 10.1248/bpb.28.1795. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- He Q, Titley H, Grasselli G, Piochon C, Hansel C. Ethanol affects NMDA receptor signaling at climbing fiber-Purkinje cell synapses in mice and impairs cerebellar LTD. J Neurophysiol. 2013;109:1333–1342. doi: 10.1152/jn.00350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR, Proctor WR, Lombroso PJ, Browning MD. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci USA. 2011;108:6650–6655. doi: 10.1073/pnas.1017856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Jaffe D, Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. J Neurophysiol. 1990;64:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Johnston D, Williams S, Jaffe D, Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- Kim DH, Jeon SJ, Jung JW, Lee S, Yoon BH, Shin BY, Son KH, Cheong JH, Kim YS, Kang SS, Ko KH, Ryu JH. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur J Pharmacol. 2007;574:140–147. doi: 10.1016/j.ejphar.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Nakajima S, Toyama K. Induction of long-term potentiation without participation of N-methyl-D-aspartate receptors in kitten visual cortex. J Neurophysiol. 1991;65:20–32. doi: 10.1152/jn.1991.65.1.20. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Li M, Lu Y, Hu Y, Zhai X, Xu W, Jing H, Tian X, Lin Y, Gao D, Yao J. Salvianolic acid B protects against acute ethanol-induced liver injury through SIRT1-mediated deacetylation of p53 in rats. Toxicol Lett. 2014;228:67–74. doi: 10.1016/j.toxlet.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Liguori A, Robinson JH. Caffeine antagonism of alcohol-induced driving impairment. Drug Alcohol Depend. 2001;63:123–129. doi: 10.1016/S0376-8716(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lu KH, Liu CT, Raghu R, Sheen LY. Therapeutic potential of chinese herbal medicines in alcoholic liver disease. J Tradit Complement Med. 2012;2:115–122. doi: 10.1016/s2225-4110(16)30084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Spinetta MJ, Woodlee MT, Feinberg LM, Stroud C, Schallert K, Cormack LK, Schallert T. Alcohol-induced retrograde memory impairment in rats: prevention by caffeine. Psychopharmacology (Berl.) 2008;201:361–371. doi: 10.1007/s00213-008-1294-5. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Nie Z, Criado JR, Siggins GR. Ethanol inhibition of N-methyl-D-aspartate responses involves presynaptic gamma-aminobutyric acid(B) receptors. J Pharmacol Exp Ther. 2000;294:637–647. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park HJ, Kim BC, Kim DH, Ryu JH. Evidences of the role of the rodent hippocampus in the non-spatial recognition memory. Behav Brain Res. 2016;297:141–149. doi: 10.1016/j.bbr.2015.10.018. [DOI] [PubMed] [Google Scholar]