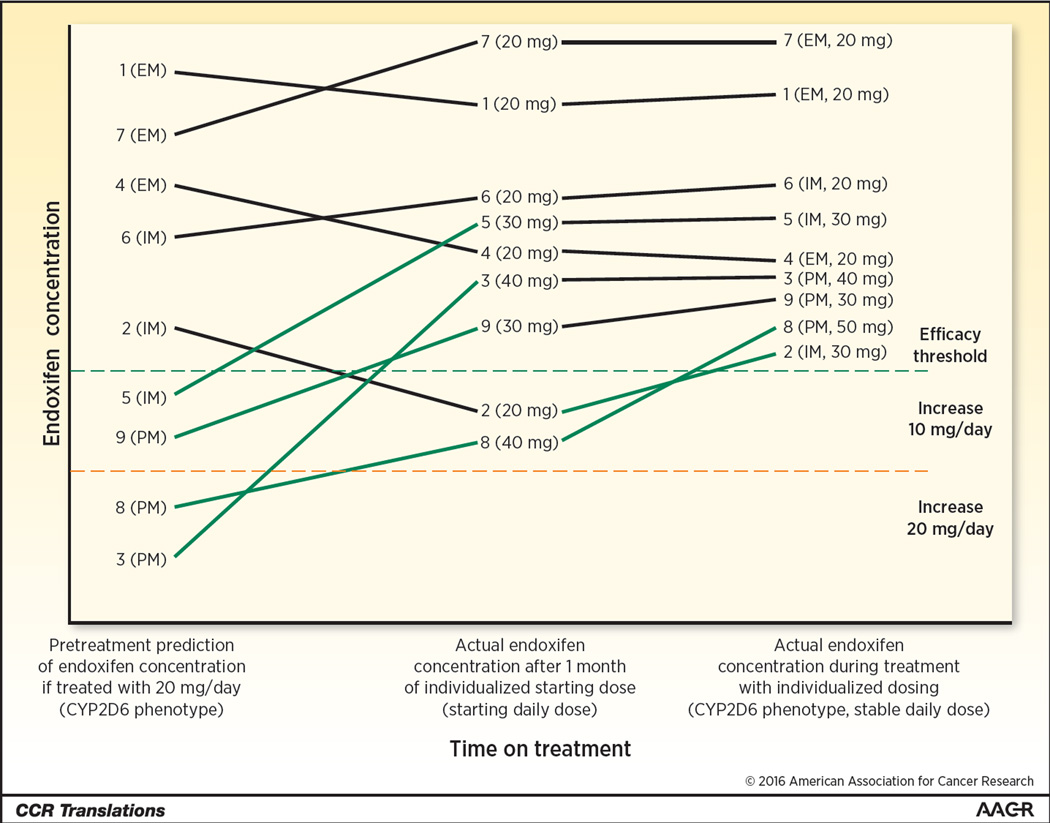
Figure 1. Illustration of 9 patients matriculating through an integrated personalized treatment strategy.
Baseline information including CYP2D6 phenotype (poor (PM), intermediate (IM), or extensive (EM) metabolizer) and clinical information (body mass index, drug-drug interactions, etc.) are used to predict endoxifen concentration during treatment with the typical dose of 20 mg/day. Patients predicted to be above the efficacy threshold (hatched green horizontal line) are started at the typical dose. Patients predicted to be below the threshold are pre-emptively dose escalated by 10 or 20 mg/day. After 1 month of treatment, endoxifen concentration is measured to determine whether further dose personalization is necessary. Some patients who were not expected to require escalation (i.e. #2) have sub-therapeutic concentration and require a dose increase. Other patients who had a dose escalation (i.e. #8) require further escalation to reach therapeutic endoxifen levels. Ultimately, all patients achieve therapeutic endoxifen concentrations, maximizing tamoxifen treatment effectiveness.