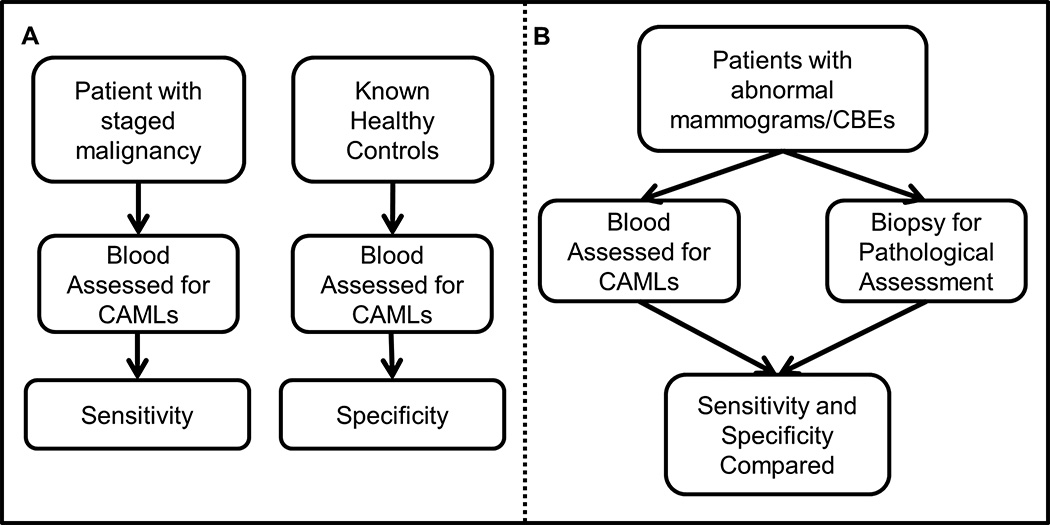
Figure 1. Overview of sensitivity and specificity determination based on our two studies, either Study A: populations of women with diagnosed breast cancer or Study B: populations of women with positive Clinical Breast Exam (CBE)/mammography but before diagnosis.
(A) Patients with invasive breast malignancy were used to determine sensitivity of an assay based on CAML presence. Healthy controls were used to determine the specificity. (B) The assay was then tested using patients with positive breast mammography, or positive CBEs, for CAML presence by standard blood draw at the time core biopsy was taken. Standard pathological assessment was used to determine invasive malignant disease, benign condition, or non-invasive disease/Stage 0. Patient information was then unblinded and sensitivity/specificity of CAMLs in relation to pathological assessment was compared.