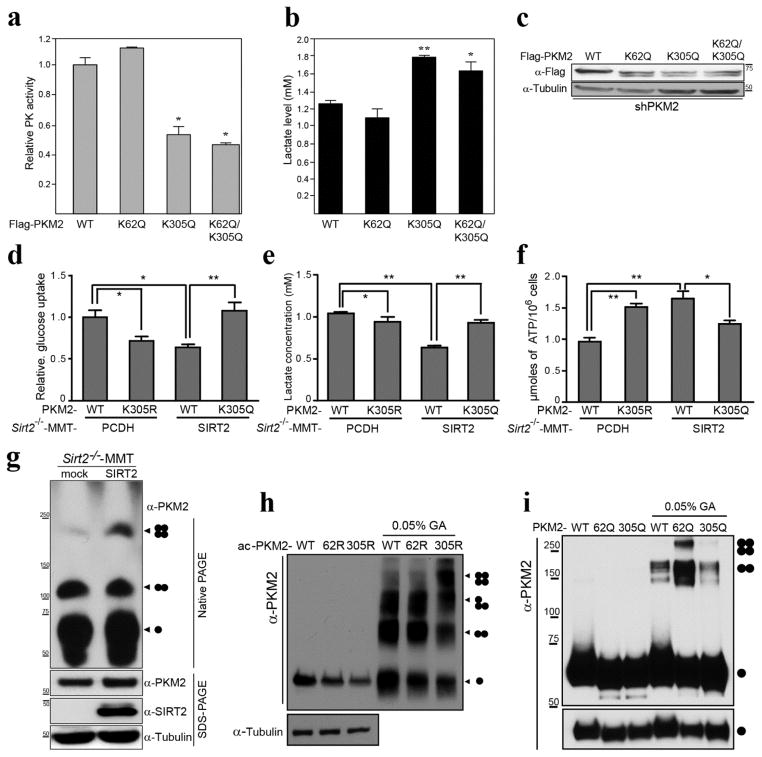
Figure 4. Acetylation status of K305 directs PKM2 activity.
(a–c) HeLa cells were infected with shPKM2 and were subsequently transiently transfected with Flag-PKM2 and the PKM2 mutant acetylated mimic expression vectors Flag-PKM2K62Q, Flag-PKM2K305Q, Flag-PKM2K62Q/K305Q). (a) Pyruvate kinase activity and (b) lactate production assay were performed using eluted PKM2-WT protein as well as protein from cells transfected with the PKM2 mutants. * indicates P < 0.05 and ** indicates P < 0.01. (c) Extracts from the shPKM2 cells transfected with these expression vectors were immunoblotted with anti-Flag and tubulin antibodies. (d–f) PKM2 was knocked down in Sirt2−/−-MMT cells were subsequently transfected with Flag-PKM2WT, Flag-PKM2K305R, and Flag-PKM2K305Q. (d) Glucose uptake, (e) lactate production and (f) ATP production assays were performed. (g) Sirt2−/−-MMT cells were infected with lenti-SIRT-WT, and control and SIRT2-expressing stable cells were lysed in native page sample buffer, separated by native-PAGE or SDS-PAGE, and immunoblotted with anti-PKM2, SIRT2, and tubulin antibodies. (h–i) HeLa cells were transfected with HATs (PCAF/Tip60) with either Flag-PKM2 or the PKM2 deacetylation mimic mutants, Flag-PKM2K62R and Flag-PKM2K305R (h). HeLa cells were transfected Flag-PKM2 or Flag-PKM2 acetylation mimic mutants, Flag-PKM2K62Q and Flag-PKM2K305Q (i). Cells then were lysed and acetylated PKM2 proteins were eluted by Flag peptide. Eluted proteins were cross-linked by 0.05% glutaraldehyde on ice. The samples were separated by SDS-PAGE, and immunoblotted with anti-PKM2 and tubulin antibodies.