Abstract
Background:
Cyclosporine and tacrolimus are limited by a narrow therapeutic window. Maintaining immunosuppressive drugs at desired levels may be difficult. Pharmaceutical care emerges as a philosophy of practice that enhances medication use and leads to a better control of serum concentration.
Objective:
This study aims to evaluate the impact of pharmaceutical care in the maintaining of proper serum levels of immunosuppressive medications in patients who have undergone allo-HSCT.
Methods:
The study had a quasi-experimental design that included a comparison group. The service model used was pharmacotherapy follow-up, according to an adaptation of the Dader method. The pharmacist consultation was carried out at a day-hospital or at the outpatient hematology clinic as needed. The intervention group consisted of 22 patients seen by a clinical pharmacist. The control group consisted of 44 patients that received standard care. This study aims to evaluate the impact of pharmaceutical care on keeping patient serum levels of cyclosporine and tacrolimus within the desired range.
Results:
Control group displayed 65% of the proper serum levels of immunosuppressive agents. While In intervention group, the figure was 82% (p = 0.004).
Conclusion:
The role of the pharmacist in the multidisciplinary team may contribute to a greater success in attaining the patients’ therapeutic targets with regard to the use of immunosuppressant.
Keywords: Medication Adherence, Bone Marrow Transplantation, Pharmaceutical Services, Professional Practice, Non-Randomized Controlled Trials as Topic, Brazil
INTRODUCTION
After the completion of allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) patients are vulnerable to graft versus host disease (GVHD) and complications from infections caused by viruses and bacterial disease. The prophylactic use of cyclosporine or tacrolimus reduces the occurrence of GVHD and the use of these drugs after the first signs of this disease may prevent its development.1,2,3,4
Studies demonstrate that, to obtain the desired effect, it is necessary to maintain serum levels of immunosuppressant within a narrow therapeutic window. Keeping immunosuppressive serum levels below the desired range may cause therapeutic ineffectiveness. On the other hand, levels above the desired range can lead to intoxication that put patients at risk.5
Maintaining immunosuppressive drugs at desired levels is not an easy task. There are different factors that affect their serum concentrations. Genetic factors, including Cytochrome P450 genetic polymorphisms, may influence the serum concentration of calcineurin inhibitors.6,7 Drug interactions can significantly alter the serum levels of immunosuppressants.8,9 Physiological factors such as the fluctuation of renal function may alter tacrolimus serum concentration.10 Other factors are related to patients´ behavior such as non-adherence to pharmacotherapy and the misuse of medication.11 Griva et al. found that of those with kidney transplants 25.4% failed to reach the immunosuppressive target levels. In addition, these levels were significantly associated with unintentional non-adherence to pharmacotherapy.12
Pharmaceutical care emerges as a philosophy of practice that may be useful to enhance medication use which, in turn, leads to a better therapeutic outcome. There were few studies that evaluated this practice in patients who had undergone some type of transplant. Some demonstrated beneficial effects such as an improvement in adherence to therapy13,14,15 and a reduction of transplant patient risks associated with medications errors.16 As regard to allo-HSCT, there has been no study evaluating the use of pharmaceutical care as a tool to improve treatment adherence.
This study aims to evaluate the impact of pharmaceutical care on keeping patient serum levels of cyclosporine and tacrolimus within the desired range.
METHODS
The study had a quasi-experimental design with a comparison group. The study was previously approved by the research ethics committee of the Hospital de Clínicas de Porto Alegre (IRB: 110020). The authors chose this design due to the small number of allo-HSCT carried out in the hospital.
Patient Selection
The intervention group included patients who had undergone all types of allo-HSCT regardless of age or gender. In addition, they were using tacrolimus or cyclosporine.
All patients who had undergone allo-HSCT in the hematology unit of HCPA between May 2011 and October 2012 were invited to take part in the study. During the first appointment after hospital discharge of patients were invited to participate and provide informed consent before taking part in the research study. All the patients from that time period agreed to participate and were followed for six months from the time of hospital discharge.
The control group was a historical cohort, consisted of patients who had undergone allo-HSCT three years before the start of the study. For every patient in the intervention group, two patients in the control group were selected.
Exclusion criteria: Patients who despite having undergone an allogeneic procedure developed autologous chimerism.
Location
The study was carried out at the Hospital de Clínicas de Porto Alegre (HCPA), a tertiary care facility which has carried out highly complex procedures (that require advanced technological resources and multidisciplinary medical expertise). The Hematology Service of HCPA is one of the largest in Brazil. This Hospital has been certified by Joint Commission International.
Pharmaceutical care
The service model used was pharmacotherapy follow-up, in line with an adaptation of the Dader method.17,18 The pharmacist consultation was carried out in a day-hospital or in the outpatient Hematology Clinic according to the patient´s needs. The first meeting between pharmacist and patient was always on the first day after discharge from an isolated ward.
The pharmacotherapy follow-up consisted of a review of patient pharmacotherapy according to the adapted Dader method. The service model allowed the identification of critical pharmacotherapy issues and their causes, expressed by the patient or not (Figure 1). Once the problem and its causes were detected, the pharmacist set a therapeutic goal and action strategy to be put into effect. This strategy might involve discussion of pharmacotherapy with the medical team as well as suggestion of changes in posology, in dosage or in medicine prescribed. Many of these approaches were chosen as a result of monitoring of serum levels of immunosuppressive drugs by pharmacists and the medical team. Another possible intervention considered was the educational one. In such cases, the pharmacist sought to empower the patient. This included an explanation of the effects of the drugs on the organism, their possible adverse reactions as well as the importance of treatment adherence and the consequences of non-adherence. For this task, the pharmacist used, when necessary, printed educational material to facilitate better recall of the information by patients. Before being put into practice, all interventions that directly involved patients were previously explained to them. To do so, the authors always took into account the patients´ life histories, beliefs and desires. In this way, it was possible to establish a cooperative agreement with the patients when necessary.
Figure 1.
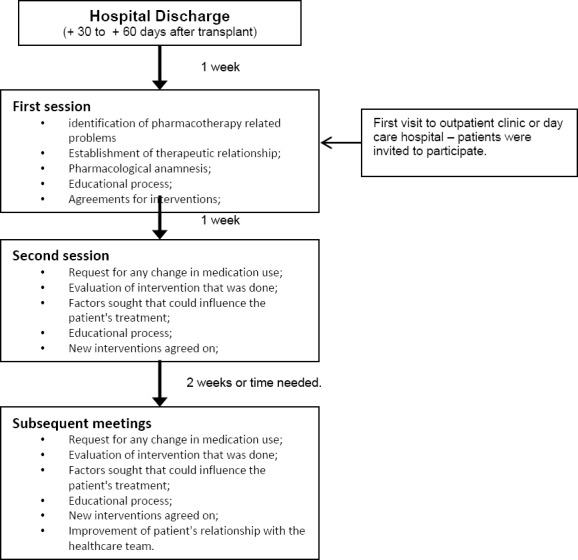
Flowchart of pharmacotherapy follow-up
Immunosuppressive serum concentration
Cyclosporine and tacrolimus were the two immunosuppressive agents examine given that these tests were carried out during routine care of these patients. The method used for the measurement of ciclosporin was monoclonal and fluorescence polarization immunoassay. While for tacrolimus, the immunoenzymatic microparticles method was used.
The levels were measured at a steady state (twelve hours after the drug was taken) according to the institution’s protocol. Only those patients who had at least six level measurements during the study period were included in this analysis. Data was collected from the patients’ medical records and the levels taken into account were those from the first 6 months after hospital discharge.
The target values used by the HCPA laboratory were 100 to 400 ng/mL for cyclosporine and 5 to 25 ng/mL for tacrolimus.
Statistical Analysis
Statistical analysis was carried out using SPSS 20 software. Categorical variables were analyzed using the Chi-square test or Fisher’s exact test, as appropriate. Analysis was done considering a significance level up to 5%.
RESULTS
Comparison of demographic characteristics and baseline clinical conditions of patients in intervention and control groups is shown in Table 1. Despite the differences, there were no statistically significant differences between the groups. In the intervention group only one patient (3%) was a native of Porto Alegre, the same city where the hospital is located. In the control group, ten patients (18%) lived in Porto Alegre. Another characteristic that differed between groups was the place where the patients resided after hospital discharge. In the intervention group, most patients (60%) moved to cities in the metropolitan region of Porto Alegre, while the control group remained in the capital (50%).
Table 1.
Patients’ demographic data.
| Characteristics | Intervention n=22 | Control n=44 | p-value |
|---|---|---|---|
| Average age/years (range) | 27.64 (5 - 58) | 29.63 (5 - 60) | 0.67 |
| Average years of study (range) | 7.64 (0 - 19) | 6.74 (0 - 15) | 0.48 |
| Male (%) | 11 (50) | 28 (64) | 0.31 |
| N° of medicines in use (range) | 10 (8 – 12) | 10 (8 - 12) | 0.99 |
| Poorest SeS (%) | 5 (22.7) | 12 (27.3) | 0.77 |
| Prophylaxis (%) | 0.99 | ||
| Cyclosporine | 12 (55) | 24(55) | |
| Tacrolimus | 10 (45) | 20 (45) | |
| Comorbidities(%) | 17 (77.3) | 31 (70.4) | 0.71 |
| Hypertension | 11 (50) | 20 (45) | |
| Depression | 5 (22.7) | 11 (25) | |
| Dislipidemia | 4 (18) | 6 (13.6) | |
| Diabetes Mellitus II | 2 (9) | 5 (11) | |
| Hepatic disorder | 2 (9) | 6 (13.6) | |
| Hormonal disorder | 2 (9) | 4 (9) | |
| Pre-Transplant Place of Residence (%) | 0.14 | ||
| Capital | 1 (4.5) | 7 (15.9) | |
| Metropolitan area | 10 (45.5) | 21 (47.8) | |
| Countryside | 8 (36.4) | 13 (29.5) | |
| Other States | 3 (13.6) | 3 (6.8) | |
| Residence After Transplant (%) | 0.74 | ||
| Capital | 9 (40.9) | 18 (15.8) | |
| Metropolitan area | 13 (59.1) | 24 (54.5) | |
| Other States | 0 | 2 (4.5) |
SeS: Socioeconomic Status; Poorest SeS was based on Silla et al.19
The intervention group consisted of 22 patients and the control group consisted of 44 patients. The number of measurements per patient ranged from 6 to 22 within six months after the beginning of the study.
The intervention group showed a mean of 15.5 serum measures per patient, compared to control group that had a mean value of 14.9 measures. Considering the total measurements of cyclosporine and tacrolimus, the control group had 65% of the levels measured within the desired range. In the intervention group the figure was 82% (p=0.004). These values are shown in Table 2.
Table 2.
Measurements levels of cyclosporine and tacrolimus in attained target.
| Intervention (n=22) | Control (n=44) | p-value | |
|---|---|---|---|
| Above target (%) | 8 (2) | 21 (3) | 0.326 |
| Attained target (%) | 280 (82) | 425 (65) | 0.004 |
| Below target (%) | 53 (16) | 208 (32) | 0.004 |
| Total measurements | 341 | 654 |
DISCUSSION
Values obtained indicated that there was an increase in the number of measurements within the desired range in the intervention group. This effect was due to pharmacist intervention.
This result is consistent with the data found in published studies for other types of transplants. In the clinical trial carried out by Klein et al., 78% of the patients in the pharmaceutical care group had immunosuppressive serum concentration within the desired range. Meanwhile, only 51% of patients in the control group reached the target (p<0,001).15 In the study conducted by Chisholm et al. 64% of patients in the pharmaceutical care group attained target immunosuppressive levels, compared to 48% in the control group (p<0,05).13
Some published studies have demonstrated the correlation between immunosuppressive serum levels and successful medication adherence.18 This facts indicates that the inclusion of a clinical pharmacist in the multidisciplinary team may be helpful to improve patients´ adherence to post-transplant pharmacological treatment.
Those who moved to from the countryside to the capital of the state had better access to medication. Thus, higher percentage of the control group moved to Porto Alegre after discharge. This improved access may have contributed to a higher number of measurements within the target range of immunosuppressive serum concentration in the control group. Consequently, the effect of the intervention was even more striking.
Limitations
The need to choose a quasi-experimental design certainly compromises the impact of the results. Unfortunately, the development of a randomized clinical trial was impractical due to the reduced number of patients that undergo allo- HSCT in HCPA every year. The dependence on clinical data is in itself a limitation. However, as the clinical protocol remained the same for all patients included in the study, the risk of potential drug interaction interference was reduced.
CONCLUSIONS
In our study, the participation of the pharmacist in the multidisciplinary team may have contributed to a greater success in achieving the patients’ therapeutic goals related to immunosuppressive therapy. This demonstrates that these professionals can surely contribute to the well-being of this patient population by improving their use of medicine in their treatment. This contribution may also be important in optimizing proper pharmacotherapy as well as its consequent enhancement of clinical parameters.
ACKNOWLEDGEMENT
The authors acknowledge the collaboration of professionals from the day hospital and the outpatient hematology clinics.
The authors also wish to thank the National Research Council (CNPq) for granting a PhD scholarship which enabled the completion of this work and the FIPE-HCPA who sponsored this project.
Footnotes
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest to disclose.
Funding: Brazilian National Research Council (CNPq)
Contributor Information
Paulo M. CORRÊA, Professor of Pharmaceutical Sciences at Universidade Federal do Amazonas. Itacoatiara (Brazil). paulomaxcorrea@gmail.com
Joice Zuckermann., Hospital de Clínicas de Porto Alegre. Porto Alegre (Brazil). Joicezuckermann@gmail.com.
Gustavo B. Fischer, Physician at Hospital de Clínicas de Porto Alegre. Porto Alegre (Brazil). gustavo_fischer@msn.com
Mauro S. Castro., Professor at School of Pharmacy at Universidade Federal do Rio Grande do Sul Brazil. Porto Alegre (Brazil). decastro.mauro@gmail.com
References
- 1.Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ. Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med. 1988;319(2):65–70. doi: 10.1056/NEJM198807143190201. [DOI] [PubMed] [Google Scholar]
- 2.Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ, Flowers ME, Chua BK, Rotta M, Storb R. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):414–422. doi: 10.1016/j.bbmt.2011.08.016. doi: 10.1016/j.bbmt.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogosheske JR, Fargen AD, DeFor TE, Warlick E, Arora M, Blazar BR, Weisdorf DJ, Brunstein CG. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival. Bone Marrow Transplant. 2014 Jan;49(1):122–125. doi: 10.1038/bmt.2013.139. doi: 10.1038/bmt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, Scarisbrick JJ, Taylor PC, Hadzic N, Shaw BE, Potter MN Haemato-oncology Task Force of British Committee for St andards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158(1):30–45. doi: 10.1111/j.1365-2141.2012.09129.x. doi: 10.1111/j.1365-2141.2012.09129.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadpour N, Elyasi S, Vahdati N, Mohammadpour AH, Shamsara J. A review on therapeutic drug monitoring of immunosuppressant drugs. Iran J Basic Med Sci. 2011;14(6):485–498. [PMC free article] [PubMed] [Google Scholar]
- 6.Lesche D, Sigurdardottir V, Setoud R, Oberhänsli M, Carrel T, Fiedler GM, Largiadèr CR, Mohacsi P, Sistonen J. CYP3A5*3 and POR*28 genetic variants influence the required dose of tacrolimus in heart transplant recipients. Ther Drug Monit. 2014;36(6):710–715. doi: 10.1097/FTD.0000000000000080. doi: 10.1097/FTD.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 7.Song J, Kim MG, Choi B, Han NY, Yun HY, Yoon JH, Oh JM. CYP3A5 polymorphism effect on cyclosporine pharmacokinetics in living donor renal transplant recipients: analysis by population pharmacokinetics. Ann Pharmacother. 2012;46(9):1141–1151. doi: 10.1345/aph.1R004. doi: 10.1345/aph.1R004. [DOI] [PubMed] [Google Scholar]
- 8.Mori T, Kato J, Yamane A, Sakurai M, Kohashi S, Kikuchi T, Ono Y, Okamoto S. Drug interaction between voriconazole and tacrolimus and its association with the bioavailability of oral voriconazole in recipients of allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2012;95(5):564–569. doi: 10.1007/s12185-012-1057-2. doi: 10.1007/s12185-012-1057-2. [DOI] [PubMed] [Google Scholar]
- 9.Leather H, Boyette RM, Tian L, Wingard JR. Pharmacokinetic evaluation of the drug interaction between intravenous itraconazole and intravenous tacrolimus or intravenous cyclosporin A in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2006 Mar;12(3):325–334. doi: 10.1016/j.bbmt.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Sumi M, Konishi H, Ikuno Y, Hoshino N, Minouchi T, Yamaji A. Change in blood tacrolimus concentration by fluctuation of renal function in a bone marrow transplant patient. Eur J Drug Metab Pharmacokinet. 2009;34(3-4):201–204. doi: 10.1007/BF03191174. [DOI] [PubMed] [Google Scholar]
- 11.Dew M, DiMartini A, Dabbs A, Myaskovsky L, Steel J, Unruh M, Switzer GE, Zomak R, Kormos RL, Greenhouse JB. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 12.Griva K, Davenport A, Harrison M, Newman SP. Non-adherence to immunosuppressive medications in kidney transplantation: intent vs. forgetfulness and clinical markers of medication intake. Ann Behav Med. 2012;44(1):85–93. doi: 10.1007/s12160-012-9359-4. doi: 10.1007/s12160-012-9359-4. [DOI] [PubMed] [Google Scholar]
- 13.Chisholm MA, Mulloyb L, Jagadeesanb M, DiPiro J. Impact of clinical pharmacy services on renal transplant patients’ compliance with immunosuppressive medications. Clin Transplant. 2001;15(5):330–336. doi: 10.1034/j.1399-0012.2001.150505.x. [DOI] [PubMed] [Google Scholar]
- 14.Mishima K, Makino K, Shimada M, Suehiro T, Oishi R. Roles of pharmacist as a member of the liver transplant team in controlling tacrolimus whole blood concentrations and monitoring adverse drug events such as convulsion. Jpn J Pharm Health Care Sci. 2002;28:393–400. doi: 10.5649/jjphcs.28.393. [Google Scholar]
- 15.Klein A, Otto G, Kramer I. Impact of a pharmaceutical care Program on liver transplant patients’compliance with immunosuppressive medication: a prospective, randomized, controlled trial using electronic monitoring. Transplantation. 2009;87(6):839–847. doi: 10.1097/TP.0b013e318199d122. doi: 10.1097/TP.0b013e318199d122. [DOI] [PubMed] [Google Scholar]
- 16.Musgravea CR, Pilch NA, Taber DJ, Meadows HB, McGillicuddy JW, Chavin KD, Baliga PK. Improving transplant patient safety through pharmacist discharge medication reconciliation. Am J Transplant. 2013;13(3):796–801. doi: 10.1111/ajt.12070. doi: 10.1111/ajt.12070. [DOI] [PubMed] [Google Scholar]
- 17.Castro MS, Fuchs FD, Santos MC, Corrêa PM, Gus M, Moreira LB, Ferreira MB. Pharmaceutical care program for patients with uncontrolled hypertension. Am J Hypertens. 2006;19(5):528–533. doi: 10.1016/j.amjhyper.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Lalić J, Veličković-Radovanović R, Mitić B, Paunović G, Cvetković T. Immunosuppressive medication adherence in kidney transplant patients. Med Princ Pract. 2014;23(4):351–356. doi: 10.1159/000362792. doi: 10.1159/000362792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silla L, Fischer GB, Paz A, Daudt LE, Mitto I, Katz B, da Graça Grossini M, Bittencourt HN, Jochims A, Fogliatto L, Bittar CM, Friedrisch JR, Bittencourt RI. Patient socioeconomic status as a prognostic factor for allo-SCT. Bone Marrow Transplant. 2009;43(7):571–577. doi: 10.1038/bmt.2008.358. doi: 10.1038/bmt.2008.358. [DOI] [PubMed] [Google Scholar]


