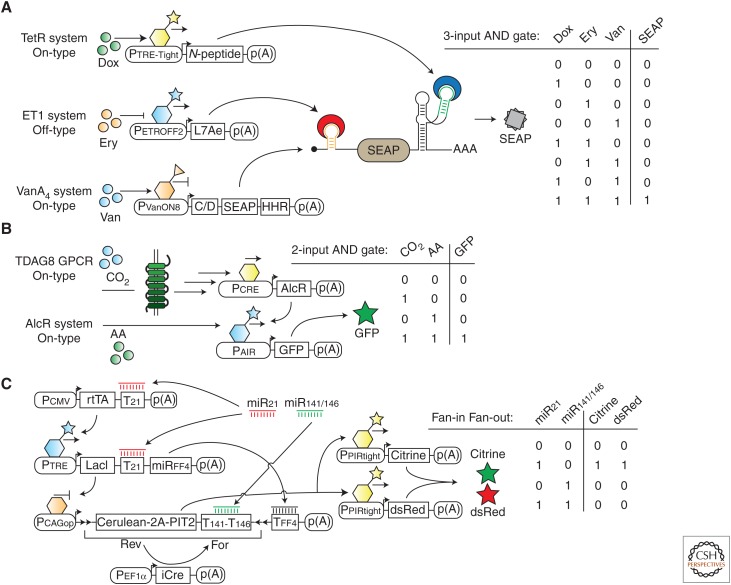
Figure 3.
Engineered gene circuits. (A) The three ligand-responsive, transcriptional gene-control systems based on the transcriptional regulators TetR, ET1, and VanA4 are combined with two posttranscriptional gene switches that use C/D RNA motif-L7Ae protein and N-peptide-responsive ribozyme-N-peptide interactions to assemble a three-input AND gate. Mammalian cells engineered with this gene circuit can be programmed with the three ligands erythromycin (Ery), doxycycline (Dox), and vanilic acid (Van) to control output gene expression as shown in the truth table. (B) Design of a two-input AND gate that can be controlled by the two gaseous molecules carbon dioxide (CO2) and acetaldehyde (AA). Activation of the G protein–coupled receptor (GPCR) TDAG8 by low pH triggers a signaling pathway that leads to the expression of AlcR, which binds to its cognate promoter exclusively in the presence of AA, thus producing the output protein GFP. (C) Transcriptional activators (rtTA and PIT2) and repressors (LacI), in addition to the recombinase iCre, are connected to build a gene circuit using bow-tie architecture. Two miRNA inputs (miR21 and miR141/146) manipulate the gene circuit to express the two output proteins in the exclusive presence of miR21.