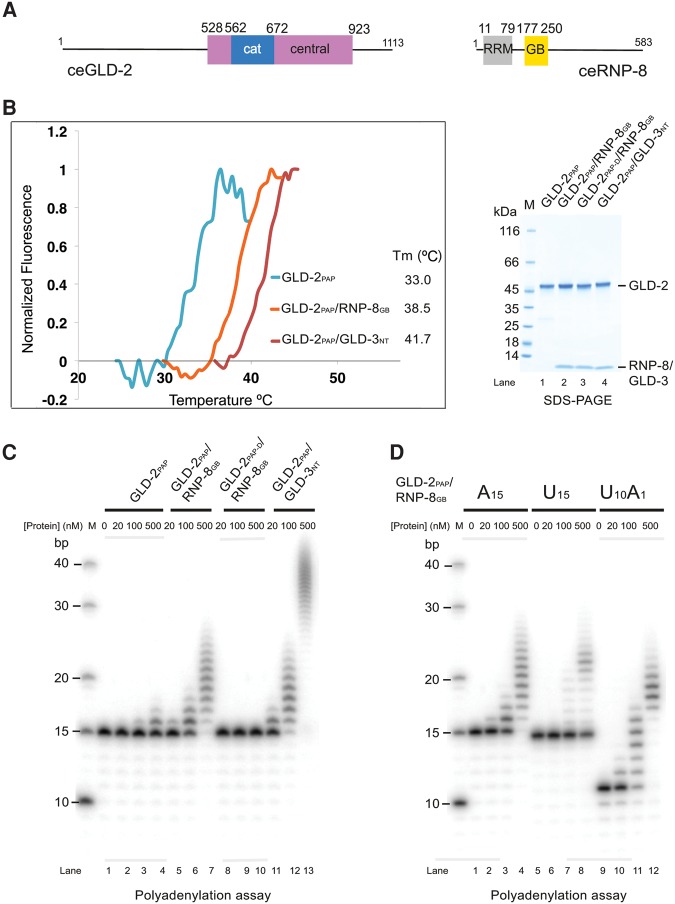
FIGURE 1.
Activity of a minimal GLD-2–RNP-8 complex. (A) Schematic representation of the domain structure of C. elegans GLD-2 and RNP-8. Globular domains are shown as rectangles and low-complexity sequences as lines. The catalytic (cat) and central domains of GLD-2 are colored in blue and pink, respectively. As in other nucleotidyltransferases, the central domain is composed of two noncontiguous polypeptide segments, which correspond in the structure to helix α1 and helices α4–α8 for the segments preceding and following the catalytic domain, respectively (see also Fig. 2). The RNA recognition motif (RRM) and the GLD-2-binding (GB) domains of RNP-8 are colored in gray and yellow, respectively. (B) Protein stability of GLD-2PAP–RNP-8GB, GLD-2PAP–GLD-3NT, and GLD-2PAP as determined by thermofluor experiments. The normalized curves and the corresponding melting temperatures are shown on the left. The Coomassie-stained 4%–15% Bio-Rad TGX SDS-PAGE gel of the proteins used in the thermofluor and poly(A) polymerase assays (C,D) are shown on the right and in Supplemental Figure 1C. (C) Polyadenylation assay of C. elegans GLD-2PAP or GLD-2PAP-D in complex with either RNP-8GB or GLD-3NT (Nakel et al. 2015). (D) Polyadenylation assay of GLD-2PAP–RNP-8GB (0, 20, 100, and 500 nM) in the presence of 5′-32P-labeled A15, U15, or U10A1 oligomers (100 nM).