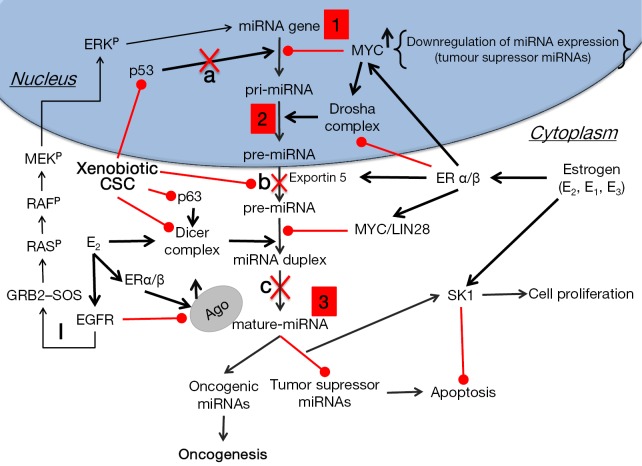
Figure 1.
Disruption of miRNA biogenesis by estrogens and cigarette smoke can lead to oncogenesis. 1, altered miRNA expression levels. Epigenetic changes/mutations in miRNA genes, and in genes responsible for miRNA transcription (insertions, deletions, translocations, inversions); 2, miRNA-processing machinery disruption. Alteration in the primary sequence of the pri-miRNA & RNAase III enzymes; 3, altered mRNA translation efficiency. With point mutation in mature miRNA and mRNA target sequence. The loss of tumor suppressor miRNAs may increase the translation of oncogenes and, thus, the formation of oncogenic proteins. By contrast, up-regulation of oncogenic miRNAs may block tumor suppressor genes that further enhance tumor development; a, in response to DNA damage, the p53/miRNA interconnection modifies the expression of miRNA genes in the nucleus; b, metabolites of environmental carcinogens bind to nucleophilic sites of miRNA precursors thus forming miRNA adducts, which cannot access the catalytic pockets of DICER in cytoplasm; c, metabolites of environmental carcinogens bind to DICER in the proximity of miRNA catalytic sites thus blocking maturation of miRNA precursors; I, Activation of EGFR by ERα or β leads to phosphorylation (P) of the adaptor protein Shc, which in turn associates with the GRB2–SOS complex, activating the Ras/Raf/MAPK pathway. The activated ERK (p44/p42 MAPK) then migrates to the nucleus where it activates the transcription of genes that promote proliferation and invasion of NSCLC cells. Lung cancer cells predominantly express ERβ (88). CSC, cigarette-smoke condensate; EGFR, EGF receptor; ER, estrogen receptor; ERK, extracellular signal-regulated kinase; GRB, growth factor receptor-bound protein; SOS, Son of Sevenless.