Abstract
Background and Purpose: Central and peripheral 5-hydroxytryptamine (5-HT) receptors play a critical role in regulation of micturition reflex. The aim of this study was to evaluate effect of a 5-HT7 receptor agonist, LP-211 (N-(4-cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide) on micturition reflex in acute spinal cord-injured (SCI) rats during infusion of vehicle into the bladder. Methods: SCI was induced by compressing T10 segment using an aneurysm clip, extradurally in male rats. Following two weeks, LP-211 doses (0.003-0.3 mg/kg) were administered cumulatively (intraperitoneally, i.p.) with 20 min interval. The 5-HT7 antagonist, SB-269970 ((R)-3-[2-[2-(4-Methylpiperidin-1-yl) ethyl] pyrrolidine-1-sulfonyl] phenol hydrochloride), was administered after achievement of LP-211 dose-response. A cystometric study was performed 2 weeks after spinal crushing in all the animals. Cystometric variables consisting of micturition volume (voided volume), residual volume (volume remaining in the bladder after voiding), and bladder capacity (micturition volume plus residual volume). Voiding efficiency was calculated as the percent of micturition volume to bladder capacity. Findings: Intact and sham-operated rats showed few significant changes in micturition reflex. SCI rats responded to LP-211 (0.003-0.3, mg/kg, i.v.) with dose-dependent increases in bladder capacity, and residual volume. In this treatment group, LP-211 induced significant dose-dependent increases in micturition volume, resulting in significant increases in voiding efficiency (P<0.001) compared to intact and sham-operated rats, SB-269970 (0.1 mg/kg, i.v.) completely reversed the LP-211-induced changes on micturition volume and voiding efficiency was decreased significantly. Conclusion: The 5-HT7 receptors activation by LP-211 facilitated the micturition reflex. Furthermore, 5-HT7 receptors do seem to play an important role in physiological regulation of micturition, and as a result, may represent a new strategy to improve voiding efficiency after SCI in patients in the future perspective.
Keywords: Spinal cord injury, 5-HT7, micturition, LP-211, rat
Introduction
Spinal cord injury (SCI) results in lower urinary tract (LUT) dysfunction that contributes to profoundly limit their quality of life. Also, it markedly relates to patients’ morbidity and mortality. Currently, there are few options to treat LUT dysfunctions [1-5]. Most patients are limited to use of urinary drainage catheters transiently or permanently. The altered voiding dynamics, repeated use of catheters, and frequent exposure to antibiotic agents predispose individuals to recurrent episodes of urinary tract infection often with resistant organisms [6-9].
The functions of LUT to store and periodically release urine are dependent on a complex neural control system in the brain and the spinal cord that utilizes multiple neurotransmitters to regulate activity of three sets of peripheral nerves: lumbosacral parasympathetic and sympathetic nerves innervating the bladder and urethral smooth muscle and pudendal nerves innervating the striated muscle of the external urethral sphincter [10-14]. Immunohistochemical, anatomical and pharmacological experiments have provided evidence that serotonin (5-hydroxytryptamine, 5-HT) is involved in the central neural pathways controlling the LUT [15-17]. Dahlström and Fuxe (1964, 1965) discovered that sympathetic and parasympathetic nuclei throughout the spinal cord exhibited a dense collection of 5-HT containing nerve fibers that originate in the raphe nuclei in the caudal brain stem [18,19].
Pharmacological and physiological studies in animals have revealed that administration of 5-HT receptor agonists or antagonists modulates reflex bladder and urethral sphincter activity [16,20-27]. These effects are mediated by activation of multiple 5-HT receptors, which are distributed at various sites in the central pathways controlling the lower urinary tract [15,27,29-33].
There are few reports on role of 5-HT7 receptor in control of micturition reflex in rats. For example, there is a report on role of intravenous 5-HT7 receptor agonist, LP44, or antagonist, SB-269970, controlling micturition reflex in chronic SCI in rats [34]. In the present study, effects of different cumulative concentrations of a 5-HT7 receptor agonist, LP-211 on micturition reflex were examined in acute spinal cord-injured male rats.
Materials and methods
Ethics and animals
All the experimental protocols were approved by the local animal care committee in accordance with Tehran University of Medical Sciences guidelines for the care and use of laboratory animals. 60 adult male Wistar albino rats (Pasteur Institute, Tehran, Iran), weighing 300-350 g with 7-8 weeks old were utilized. The animals were kept in individual propylene cages under standard laboratory conditions. Rats were maintained on a 12 h light/dark cycle at 23 ± 2°C and 50 ± 5% humidity. The animals were kept in standard room conditions and fed with standard rat diet and water ad libitum.
Chemicals and treatment
LP-211 (N-(4-cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide) and SB-269970 ((2R)-1-[(3-hydroxyphenyl) sulfonyl]-2-[2-(4-methyl-1-piperidinyl) ethyl] pyrrolidine) (both from (SERVA Chemical Co., New York)) were purchased from SERVA Chemical Co.
The drugs were dissolved in distilled water. Successive doses were administered cumulatively in a total 100 min time-course at short intervals (20 min maximum). The 5-HT7 receptor antagonist SB-269970 was administered intraperitoneally (i.p.) (0.1 mg/kg) after a dose-response study for the 5-HT7 receptor agonist, LP-211 (0.003-0.3 mg/kg, i.p.). The drugs doses were in accordance with dosages determined in previous studies [27,34,35]. All the drug solutions were administered in a volume of 0.5 ml.
Laminectomy procedures
All the surgery was carried out in sterilized condition. Animals were anesthetized by i.p. injection of ketamine hydrochloride (75 mg/kg) and xylazine hydrochloride (5 mg/kg) mixture. Their dorsal regions were shaved and cleaned with povidone Iodine 10%. Under sterile technique, after a dorsomedial incision on skin, laminectomy was performed between vertebral levels T9-T11 to expose the underlying T10 spinal cord, and the spinal cord was exposed microsurgically. After the incision of the dermal and sub-dermal tissues at the midline, paravertebral muscles were dissected bluntly exposing the lamina bilaterally. Complete laminectomy was performed, exposing the spinal cord at T9-T11. Strict bleeding control was performed using bone wax and bipolar coagulator. The crushing or SCI was induced by compressing T10 segment using an aneurysm clip, extradurally (Yasargil FE 760) for 1 min with a closing force of 180 g on the spinal cord at room temperature in T10 level [34,35]. After carefully removing the aneurysm clip, the fascia and the skin were sutured separately using silk stitches. For the sham-operated-operation, only the skin and muscles in the thoracic vertebral level were removed and the dura was kept intact. Following surgery and or/upon SCI, animals received antibiotics enrofloxacin 2.5 % intramuscular in the dose of 2.5 ml/Kg for three days. Until termination of the experiment the welfare of the rats was routinely checked.
Experimental groups
The rats were randomly divided into six groups of ten as follows:
Group 1: Intact (saline as vehicle): No SCI or treatment was performed. Samples were obtained to determine baseline biochemical values.
Group 2: Intact (LP-211 + SB269970): No SCI or treatment was performed. Samples were obtained to determine baseline biochemical values.
Group 3: Sham-operated (laminectomy + vehicle): rats underwent only a simple laminectomy. No SCI or treatment was performed.
Group 4: Sham-operated (laminectomy + LP-211 + SB269970): rats underwent a simple laminectomy and treatment. No SCI was performed.
Group 5: Treatment (laminectomy + spinal trauma + vehicle): Laminectomy and trauma was performed. Rats were received vehicle immediately following SCI.
Group 6: Treatment (laminectomy + spinal trauma + LP-211 + SB269970): Laminectomy and trauma was performed. Drugs were diluted with sterile saline and given i.p.
Cystometrograms (CMG) method and urodynamic recordings
All the rats underwent a comprehensive urodynamic evaluation at 2 weeks postoperatively as a terminal procedure. The functional assessments of the lower urinary tract included CMG recordings. For this purpose, 1 hr before start of the surgical procedures, rats was deeply anesthetized. General cystometric methods were similar to those previously described [23,34,35].
The urinary bladder was exposed via a midline abdominal incision, a polyethylene catheter (PE-90) with a flared end was inserted through the bladder dome, and a suture was tightened around it. The other end of the bladder catheter was connected to a syringe pump (model 975; Harvard Apparatus, South Natick, MA) for continuous infusion of saline.
Physiological saline was infused at room temperature into the bladder to elicit repeated voiding responses. The infusion rate was 0.11-0.15 ml/min for spinally intact rats and 0.30-1.10 ml/min for spinal cord-injured rats (similar to Ref. [19]); the higher rate of infusion is necessary in spinal cord-injured rats with their enlarged bladders to ensure the occurrence of a voiding contraction within 10 min of drug administration. The voided fluid was collected in a syringe to determine the micturition volume.
The analyzed cystometric variables consisting of micturition volume (voided volume), residual volume (volume remaining in the bladder after voiding), and bladder capacity (micturition volume plus residual volume). Voiding efficiency was calculated as the ratio of micturition volume to bladder capacity and expressed as a percent.
Statistical analysis
The analyses were performed using SPSS (v. 18). The results are given as means ± SE. Differences between groups were assessed using a one-way analysis of variance (ANOVA) and followed by Tukey post-hoc test. The values of P < 0.05, was considered statistically significant.
Results
After infusing of saline into the bladder, urodynamic studies of the bladder activity, including bladder capacity, micturition volume, and residual volume were
Effects of intraperitoneal administration of LP-211 in SCI (n = 20) and intact rats (n=20) and sham operated animals (n=20) at 2 weeks post-operatively were investigated. The urodynamic studies were performed after the administration of increasing doses of LP-211 at 0.003, 0.01, 0.03, 0.1 and 0.3 mg/kg. Comprehensive sets of urodynamic recordings were obtained within 20 minutes after each drug administration.
The experimental protocol was modified so that each dose of LP-211 was given immediately before performing a single filling cystometrogram (CMG). Dose-response curves for cystometric variables in SCI, intact and sham operated rats (n =60) are shown in Figures 1, 3 and 5.
Figure 1.
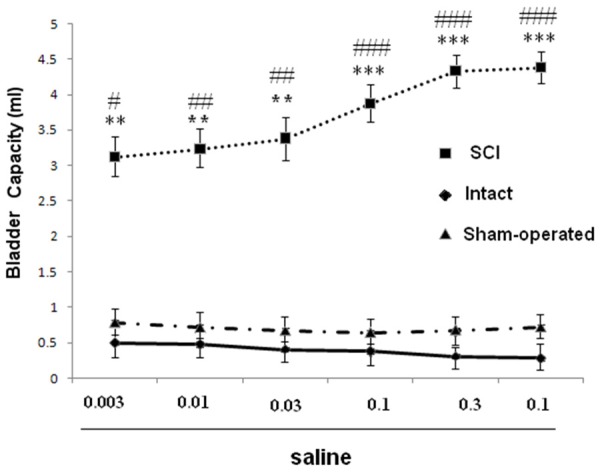
Dose-response curves for effects of saline (mg/kg, i.p.) on bladder capacity in SCI, intact and sham rats. **P < 0.01 and ***P < 0.001 indicate a statistically significant difference comparing intact and SCI. ##P < 0.01 and ###P < 0.001 indicate a statistically significant difference comparing sham and SCI.
Figure 3.
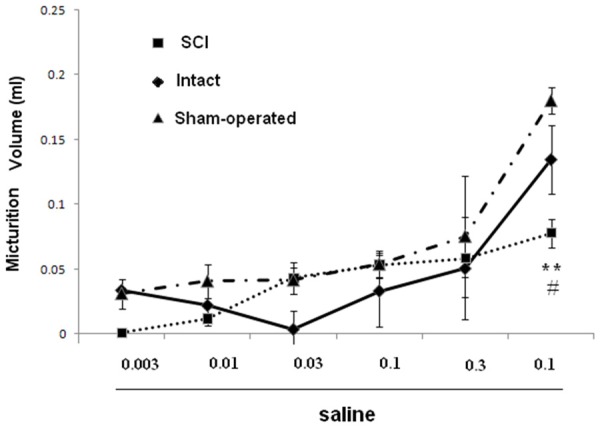
Dose-response curves for effects of saline (mg/kg, i.p.) on micturition volume in SCI, intact and sham rats. *P < 0.05 indicates a statistically significant difference comparing intact and SCI.
Figure 5.
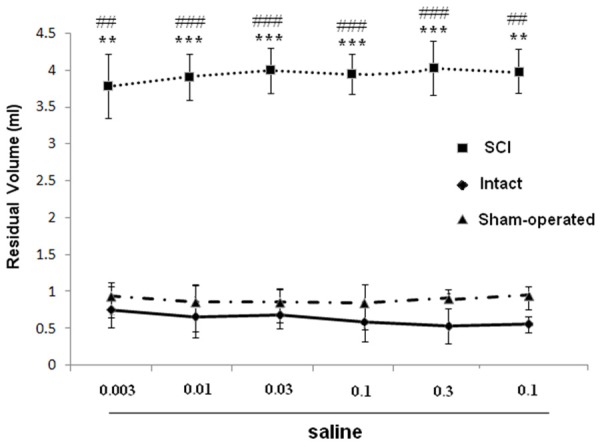
Dose-response curves for effects of saline (mg/kg, i.p.) on bladder capacity in SCI, intact and sham rats. **P < 0.01 and ***P < 0.001 indicate a statistically significant difference comparing intact and SCI. ##P < 0.01 and ###P < 0.001 indicate a statistically significant difference comparing sham and SCI.
Dose-response curves for CMG variables in rats are shown in Figure 2. With increasing LP-211 doses, bladder capacity, micturition volume, and residual volume increased significantly (P < 0.001, Figures 2, 4 and 6).
Figure 2.
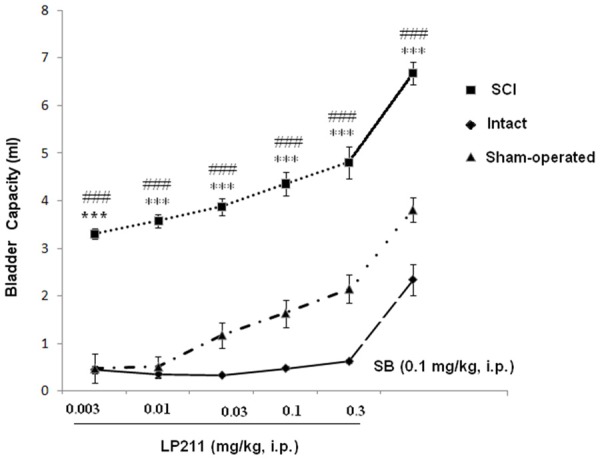
Effect of antagonist SB-269970 (0.1 mg/kg, i.p.) on dose-response effects of LP-211 (mg/kg, i.p.) on bladder capacity in SCI, intact and sham rats. ***P < 0.001 indicates a statistically significant difference comparing intact and SCI. ###P < 0.001 indicates a statistically significant difference comparing sham and SCI.
Figure 4.
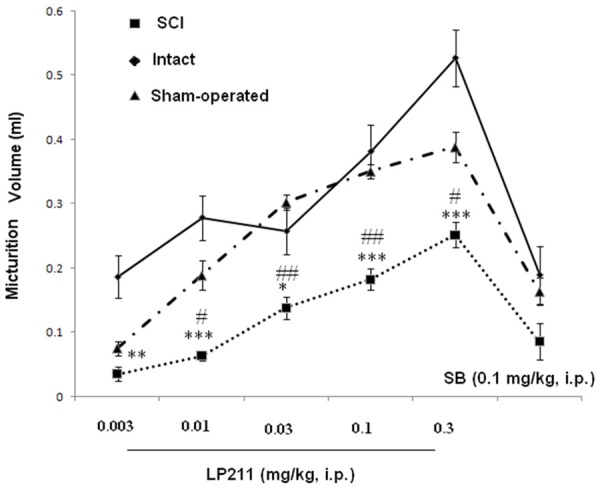
Effect of antagonist SB-269970 (0.1 mg/kg, i.p.) on dose-response effects of LP-211 (mg/kg, i.p.) on micturition volume in SCI, intact and sham rats. *P < 0.05, **P < 0.01, **P < 0.01 and ***P < 0.001 indicate a statistically significant difference comparing intact and SCI. #P < 0.05 and ##P < 0.001 indicate a statistically significant difference comparing sham and SCI.
Figure 6.
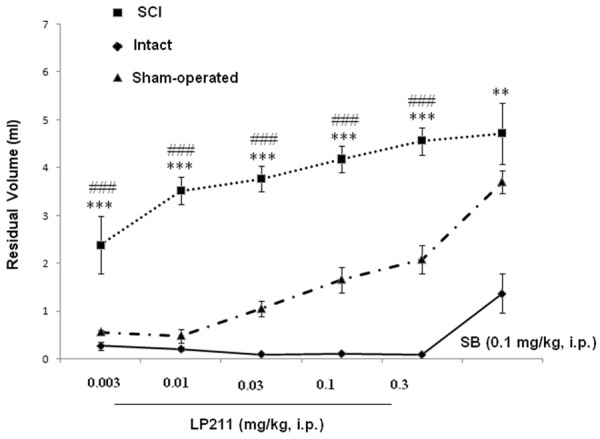
Effect of antagonist SB-269970 (0.1 mg/kg, i.p.) on dose-response effects of LP-211 (mg/kg, i.p.) on micturition volume in SCI, intact and sham rats. *P < 0.05, **P < 0.01, **P < 0.01 and ***P <0.001 indicate a statistically significant difference comparing intact and SCI. #P < 0.05 and ##P < 0.001 indicate a statistically significant difference comparing sham and SCI.
Micturition volume increased from 0.03 ± 0.03 ml (0.003 mg/kg) to 0.24 ± 0.09 ml at 0.3 mg/kg. Furthermore, considering all 40 rats together, the change from LP-21 infusion of the bladder was associated with statistically significant increases in bladder capacity from 3.96 ± 0.51 ml to 4.82 ± 0.84 ml at 0.3 mg/kg, and residual volume from 2.39 ± 0.6 ml to 4.2 ± 1.2 ml at 0.3 mg/kg.
The 5-HT7 antagonist SB-26997 was administered following completion of the LP-211 dose-response study. Micturition volume was reversed by treatment with the 5-HT7 receptor antagonist SB-26997 at 0.1 mg/kg i.p. However, changes in bladder capacity were not significant in any rat with SB-26997. In addition, we noticed that with increasing LP-211 doses, reduction of the first micturition in SCI, intact and sham rats were seen (Figures 7 and 8).
Figure 7.
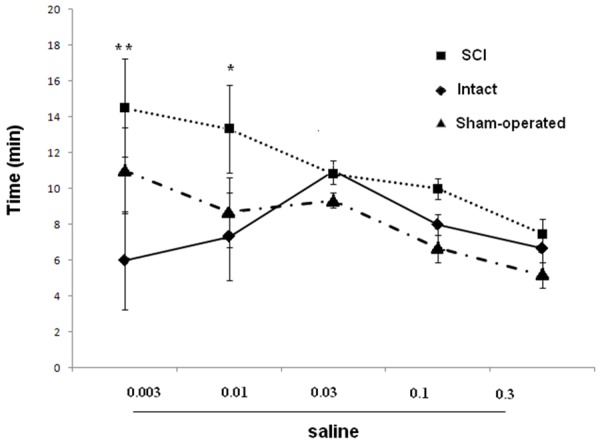
Dose-response curves for effects of saline (mg/kg, i.p.) on first urine drop micturition in SCI, intact and sham rats. *P < 0.05 indicates a statistically significant difference comparing intact and SCI.
Figure 8.
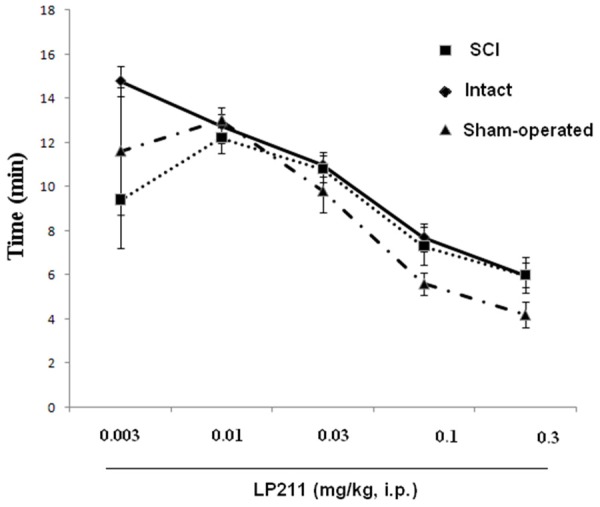
Dose-response curves for effects of LP-211 (mg/kg, i.p.) on first urine drop micturition in SCI, intact and sham rats.
Discussion
In recent years considerable efforts have been focused on development of selective 5-HT7 receptor agonists and antagonists [36,37]. Recently, we showed that acute additive LP-211 treatments in SCI group led to hyponatremia, hyperkalemia and hypermagnesemia. We stated that LP-211 might be a promising candidate for treating SCI complications in some systems especially urinary tract, nevertheless, further studies would be needed to clarify its benefits or drawbacks [38].
The results of the present study indicated that the 5-HT7 receptor agonist, LP-211, improves the bladder capacity, residual volume, and promotes micturition reflex activity, thereby improving voiding efficiency in SCI rats under anesthetization. These effects may be attributable to an action on multiple physiological mechanisms that involve endogenously released of 5-HT.
In addition, this study adds to the growing evidence, the importance of the 5-HT7 in control of micturition reflex. Nevertheless, it is concluded that the selective 5-HT7 receptor antagonist, SB-269970 inhibit the micturition reflex completely blocking central 5-HT7 receptors. In parallel, Gang W et al., reported that SB-269970 a selective 5-HT7 receptor antagonist (0.1 mg/kg, i.v.) partially or completely reversed the effects of LP-44 as 5-HT7 receptor agonist on micturition in SCI rats [34]. Moreover, in other study, Chen J et al., examined the effects of the 5-HT2A/2C receptor agonist DOI on micturition in SCI rats, so that, this effect was reversed by selective 5-HT2A/2C antagonist Ketanserin [35]. In addition, multiple previous pharmacological studies have demonstrated that micturition effects of 8-OH-DPAT in the rat were reversed by administration of 5-HT1A receptor antagonist, WAY-100635 [20,33,39-41].
On the other hand, our study demonstrates that LP-211 may reverse impaired micturition reflex function in SCI. Therefore, increasing activation of 5-HT7 receptors may represent a potentially useful therapeutic approach for reversing LUT dysfunction after SCI in rat.
The present experiment improve and extend previous studies which revealed that LP-44 enhances and SB-269970 suppresses long-latency external urethral sphincter (EUS) reflex activity evoked by stimulation of afferent axons in the pelvic nerve [34]. It was proposed that this reflex is mediated by different central pathways and have different functions. The long-latency reflex is dependent on supraspinal mechanisms and related to bursting EUS electromyographic (EMG) activity occurring during micturition. It is probable that a convergence of 5-HT and glutamatergic pathways is necessary for the regulation of EUS activity.
Furthermore, bladder activity is regulated by multiple excitatory central neurotransmitter mechanisms (glutamatergic, noradrenergic, dopaminergic, and serotonergic), as well as GABAergic and glycinergic inhibitory mechanisms [42,43]. It has believed that glutamate is the essential excitatory transmitter and that other neurotransmitters such as 5-HT and norepinephrine exert their effects by modulating glutamatergic transmission [15,42]. Taken together, these results demonstrate that spinal 5-HT7 receptors at the T10 level have an important role in tonically modulating the efferent limb of the micturition reflex in rat. Because bladder activity during continuous CMG was also stimulated by i.p. administration of LP-211 and abolished by SB-269970, spinal 5-HT7 receptors at the T9-T11 level are likely to be involved in the control of EUS as well as bladder function. The role of serotonin in modulating the micturition reflex in the rat was described by several authors. In conscious rats, at the supraspinal level, 5-HT can enhance (via 5-HT1A, 5-HT2 and 5-HT4 receptors) the micturition reflex induced by filling [21]. In the anaesthetized female rat, it was recently shown, by using selective 5-HT7 receptor antagonists administered intravenously, that supraspinal 5-HT7 receptors are involved in control of the micturition reflex [30]. However, 5-HT1A and 5-HT7 receptors have recently been suggested to have an excitatory role in control of bladder function [44].
In vivo rat bladder, the 5-HT7 receptor antagonists can inhibit the 5-HT-induced increase of intravesical pressure, while the 5-HT3, 5-HT4 receptor antagonist had no similar effect [45]. Recio confirmed that 5-HT can relax the pig urinary bladder neck through activation of muscle 5-HT7 receptors coupled to adenylyl cyclase activation [46]. While it has been reported that intravenous injection of SB-269970 had no effect on urodynamic parameters in anesthetized female spinal intact rats, intracerebroventricular injection (i.c.v.) of 5-HT7 receptor antagonist SB-269970 could induce an increase in bladder pressure threshold and capacity, a significant decrease in distension-induced bladder contraction. At the higher doses, SB-269970 blocked the micturition reflex. It means that the superspinal 5-HT7 receptors are involved in the regulation of urinary reflex, while peripheral nervous systems such as the spinal cord of 5-HT7 receptor are unable to control the micturition reflex [30].
Conclusions
In summary, the 5-HT7 receptor agonist, LP-211, improved voiding efficiency by different functions of the bladder activity and also, this research adds to the growing evidence of the importance of the 5-HT7 in the control of micturition reflex in SCI rats, and so may represent a new strategy to improve bladder voiding efficiency after SCI in experimental studies. LP-211 activated 5-HT7 receptor and facilitated the micturition reflex, which showed some clinical relevance regarding the voiding efficiency after SCI. however, further studies are necessary to clarify the role of the 5-HT7 receptor agonist, LP-211 on bladder activity.
Acknowledgements
This study was supported by Tehran University of Medical Sciences (Grant No.2014-02/85/2527).
Disclosure of conflict of interest
None.
References
- 1.Cruz CD, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. ScientificWorldJournal. 2011;11:214–234. doi: 10.1100/tsw.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li WJ, Oh SJ. Management of lower urinary tract dysfunction in patients with neurological disorders. Korean J Urol. 2012;53:583–592. doi: 10.4111/kju.2012.53.9.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schurch B, Tawadros C, Carda S. Dysfunction of lower urinary tract in patients with spinal cord injury. Handb Clin Neurol. 2015;130:247–267. doi: 10.1016/B978-0-444-63247-0.00014-6. [DOI] [PubMed] [Google Scholar]
- 4.Schöps TF, Schneider MP, Steffen F, Ineichen BV, Mehnert U, Kessler TM. Neurogenic lower urinary tract dysfunction (NLUTD) in patients with spinal cord injury: long-term urodynamic findings. BJU Int. 2015;115:33–38. doi: 10.1111/bju.13085. [DOI] [PubMed] [Google Scholar]
- 5.Moslavac S, Dzidić I, Moslavac A, Vlahek P, Filipan Z. [Urinary tract dysfunction in spinal cord injury patients] . Lijec Vjesn. 2014;136:147–152. [PubMed] [Google Scholar]
- 6.Trautner BW, Darouiche RO. Prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;25:277–283. doi: 10.1080/10790268.2002.11753628. [DOI] [PubMed] [Google Scholar]
- 7.Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med. 2002;113:67S–79S. doi: 10.1016/s0002-9343(02)01061-6. [DOI] [PubMed] [Google Scholar]
- 8.Garcia Leoni ME, Esclarin De Ruz A. Management of urinary tract infection in patients with spinal cord injuries. Clin Microbiol Infect. 2003;9:780–785. doi: 10.1046/j.1469-0691.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Rohilla RK, Sangwan K, Siwach R, Magu NK, Sangwan SS. Bladder management methods and urological complications in spinal cord injury patients. Indian J Orthop. 2011;45:141–147. doi: 10.4103/0019-5413.77134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system. Nervous control of the urogenital system. Vol. 3. Harwood, London: 1993. pp. 227–289. [Google Scholar]
- 11.Morrison JF, Birder L, Craggs M, de Groat WC, Downie JW, Drake M, Fowler CJ, Thor KB. Neural control. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence Plymouth Health. 2005. pp. 363–422. [Google Scholar]
- 12.Fowler CJ, Griffiths D, De Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009;194:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002;59(Suppl 1):30–36. doi: 10.1016/s0090-4295(01)01636-3. [DOI] [PubMed] [Google Scholar]
- 16.Thor KB. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence. Urology. 2003;62:3–9. doi: 10.1016/s0090-4295(03)00754-4. [DOI] [PubMed] [Google Scholar]
- 17.Thor KB, Donatucci C. Central nervous system control of the lower urinary tract: new pharmacological approaches to stress urinary incontinence in women. J Urol. 2004;172:27–33. doi: 10.1097/01.ju.0000118381.04432.22. [DOI] [PubMed] [Google Scholar]
- 18.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. 11 experimentally induced changes in the intraneuronal amine levels of the bulbo spinal neuron systems. Acta Physiol Scand. 1965;64:1–36. [PubMed] [Google Scholar]
- 19.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62:1–55. [PubMed] [Google Scholar]
- 20.Kakizaki H, Yoshiyama M, Koyanagi T, De Groat WC. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1407–1413. doi: 10.1152/ajpregu.2001.280.5.R1407. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka O, Gu B, Igawa Y, Nishizawa O, Pehrson R, Andersson KE. Role of supraspinal serotonin receptors for micturition in normal conscious rats. Neurourol Urodyn. 2002;21:225–230. doi: 10.1002/nau.10043. [DOI] [PubMed] [Google Scholar]
- 22.Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM. The role of 5-HT1A receptors in control of lower urinary tract function in cats. Brain Res. 2002;946:290–297. doi: 10.1016/s0006-8993(02)02897-4. [DOI] [PubMed] [Google Scholar]
- 23.Gu B, Olejar KJ, Reiter JP, Thor KB, Dolber PC. Inhibition of bladder activity by 5-hydroxytryptamine1 serotonin receptor agonists in cats with chronic spinal cord injury. J Pharmacol Exp Ther. 2004;310:1266–1272. doi: 10.1124/jpet.103.063842. [DOI] [PubMed] [Google Scholar]
- 24.Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp Neurol. 2006;199:427–437. doi: 10.1016/j.expneurol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R224–R234. doi: 10.1152/ajpregu.00780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu B, Thor KB, Reiter JP, Dolber PC. Effect of 5-hydroxytryptamine1 serotonin receptor agonists on noxiously stimulated micturition in cats with chronic spinal cord injury. J Urol. 2007;177:2381–2385. doi: 10.1016/j.juro.2007.01.110. [DOI] [PubMed] [Google Scholar]
- 27.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT1A receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1699–1706. doi: 10.1152/ajpregu.00142.2006. [DOI] [PubMed] [Google Scholar]
- 28.Chang HY, Cheng CL, Chen JJ, De Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol. 2007;292:F1044–1053. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helton LA, Thor KB, Baez M. 5-Hydroxytryptamine2a, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey and human. Neuroreport. 1994;5:2617–2620. doi: 10.1097/00001756-199412000-00053. [DOI] [PubMed] [Google Scholar]
- 30.Read KE, Sanger GJ, Ramage AG. Evidence of the involvement of central 5-HT7 receptors in the micturition reflex in anaesthetized female rats. Br J Pharmacol. 2003;140:53–60. doi: 10.1038/sj.bjp.0705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramage AG. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol. 2006;147:S120–S131. doi: 10.1038/sj.bjp.0706504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbaki Y, Ramage AG. Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol. 2008;155:343–356. doi: 10.1038/bjp.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng CL, De Groat WC. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. Am J Physiol Renal Physiol. 2010;298:F771–8. doi: 10.1152/ajprenal.00266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gang W, Hongjian T, Jasheng C, Jiemin S, Zhong C, Yuemin X, Baojun G, Andersson KE. The effect of the 5-HT7 serotonin receptor agonist, LP44, on micturition in rats with chronic spinal cord injury. Neurourol Urodyn. 2014;33:1165–70. doi: 10.1002/nau.22463. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Gu B, Wu G, Tu H, Si J, Xu Y. The effect of the 5-HT2A/2C receptor agonist DOI on micturition in rats with chronic spinal cord injury. J Urol. 2013;189:1982–8. doi: 10.1016/j.juro.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 36.Leopoldo M. Serotonin7 receptors (5-HT7Rs) and their ligands. Curr Med Chem. 2004;11:629–661. doi: 10.2174/0929867043455828. [DOI] [PubMed] [Google Scholar]
- 37.Pittalà V, Salerno L, Modica M, Siracusa MA, Romeo G. 5-HT7 receptor ligands: recent developments and potential therapeutic applications. Mini Rev Med Chem. 2007;7:945–960. doi: 10.2174/138955707781662663. [DOI] [PubMed] [Google Scholar]
- 38.Norouzi-Javidan A, Javanbakht J, Barati F, Fakhraei N, Mohammadi F, Dehpour AR. Serotonin 5-HT7 receptor agonist, LP-211, exacerbates Na (+), K (+)-ATPase/Mg (2+)-ATPase imbalances in spinal cord-injured male rats. Diagn Pathol. 2015;10:157. doi: 10.1186/s13000-015-0397-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Testa R, Guarneri L, Poggesi E, Angelico P, Velasco C, Ibba M, Cilia A, Motta G. Effect of several 5-hydroxytrypt-amine1A receptor ligands on the micturition reflex in rats: comparison with WAY100635. J Pharmacol Exp Ther. 1999;290:1258–1269. [PubMed] [Google Scholar]
- 40.Gu B, Wu G, Si J, Xu Y, Andersson KE. Improving voiding efficiency in the diabetic rat by a 5-HT1A serotonin receptor agonist. Neurourol Urodyn. 2012;31:168–73. doi: 10.1002/nau.21182. [DOI] [PubMed] [Google Scholar]
- 41.Chang HH, Havton LA. Serotonergic 5-HT (1A) receptor agonist (8-OH-DPAT) ameliorates impaired micturition reflexes in a chronic ventral root avulsion model of incomplete cauda equina/conus medullaris injury. Exp Neurol. 2013;239:210–7. doi: 10.1016/j.expneurol.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 43.De Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakizaki H, Yoshiyama M. Role of Central 5-Hydroxytryptamine Receptors in Micturition Control. LUTS: Lower Urinary Tract Symptoms. 2009;1:S36–S39. [Google Scholar]
- 45.Palea S, Lluel P, Barras M. Involvement of 5-hydroxytryptamine (HT)7 receptors in the 5-HT excitatory effects on the rat urinary bladder. BJU Int. 2004;94:1125–1131. doi: 10.1111/j.1464-410X.2004.05114.x. [DOI] [PubMed] [Google Scholar]
- 46.Recio P, Barahona MV, Orensanz LM. 5-hydroxytryptamine induced relaxation in the pig urinary bladder neck. Br J Pharmacol. 2009;157:271–280. doi: 10.1111/j.1476-5381.2009.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


