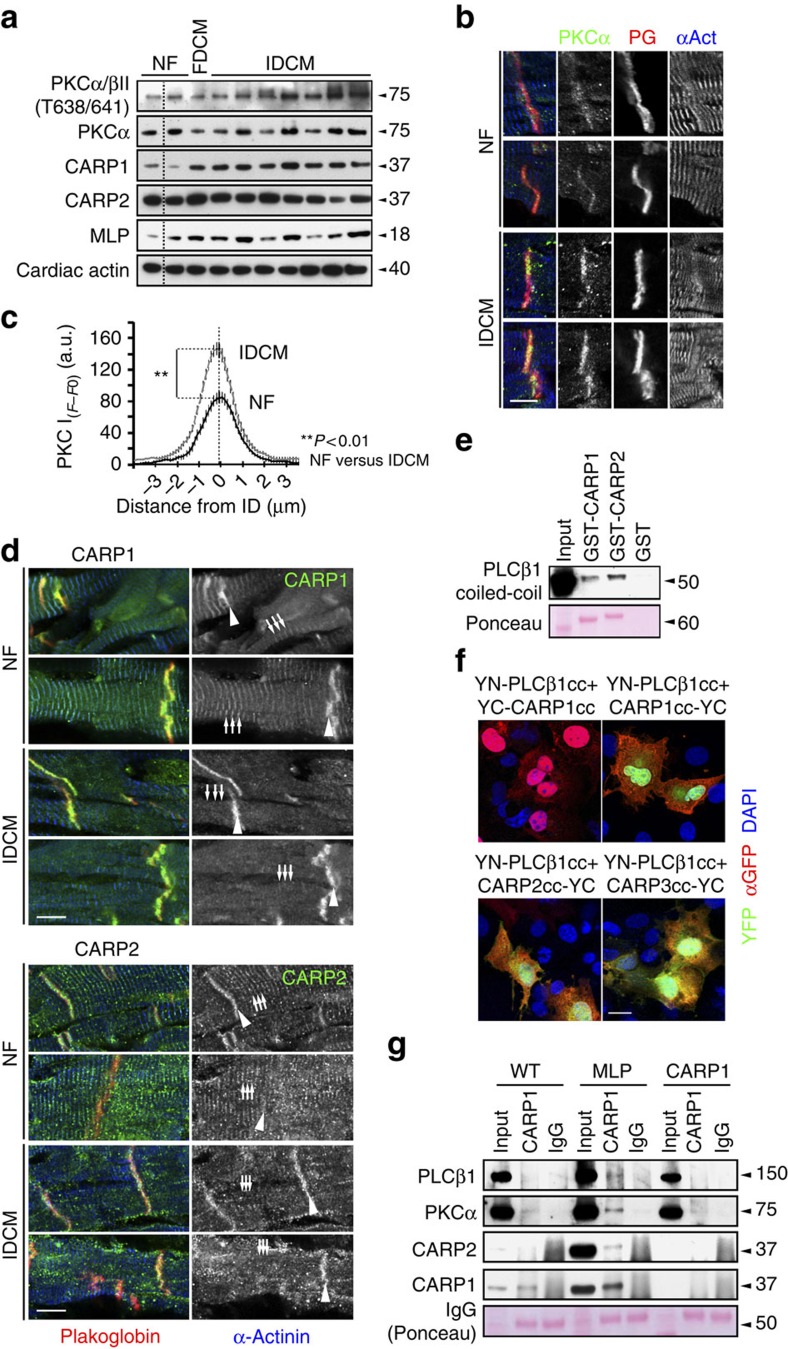
Figure 2. PKCα is sequestered in a multiprotein complex with CARP at the ID of failing hearts.
(a) Analysis of PKCα phosphorylation levels (Thr638/641), PKCα, CARP1 and CARP2 protein levels in whole extracts of NF, familial DCM (FDCM) and IDCM patient heart samples. Cardiac actin was used as a loading control. Dotted lines indicate that samples were run on the same gel, but non-consecutive. (b) Immunofluorescence staining of human NF and IDCM heart sections using antibodies against PKCα (right, green). Plakoglobin and α-actinin were used as counterstains (red and blue in overlay, respectively). Scale bar=10 μm. (c) Quantification of immunofluorescence intensity (F−F0) over IDs from (b) shows that PKCα levels at the ID are increased in IDCM samples compared to NF controls. Sample sizes for analysed ID were n=24 from three NF heart samples and n=46 from five IDCM hearts. (d) Immunofluorescence staining of human NF and IDCM heart sections using antibodies against CARP1 (top, green in overlay) and CARP2 (bottom; green in overlay). The sarcomeric association (arrows) of CARP1 and CARP2 decreases in IDCM in favour of increased ID localization (arrowheads; for quantification see Supplementary Fig. 1d,e). Plakoglobin and α-actinin were used as counterstains (red and blue in overlay, respectively). Scale bar=10 μm. (e) Pull-down assay of GFP-PLCβ1 (phospholipase-C) with GST-CARP1, GST-CARP2 or GST indicated the coiled-coil domain within PLCβ1 as minimal binding site. Ponceau stain was used to visualize GST-CARP1 and GST-CARP2 protein levels, while equal amounts of GST (not shown) were used as a control. (f) Protein complementation assay using YFP (split-fluorescent protein assay) demonstrating antiparallel association (green in overlay) between coiled-coil domains of PLCβ1 tagged on the N-terminus with a YFP N-terminal fragment (YN) and CARP1 tagged on the C-terminus with YFP C-terminal fragment (YC; upper right panel). No association was seen when CARP1 was tagged on the N-terminus with YFP C-terminal fragment (upper left panel). Split-fluorescent protein assay also demonstrated antiparallel association (green in overlay) between coiled-coil domains of PLCβ1 tagged on the N-terminus with a YFP N-terminal fragment (YN) and either CARP2 (lower left panel) or CARP3 (lower right panel) tagged on the C-terminus with YFP C-terminal fragment (YC). Transfection efficiency was validated with a GFP antibody (red in the overlay), and DAPI (blue in overlay) was used as counterstain. Scale bar=20 μm. (g) Co-immunoprecipitation of PLCβ1, PKCα, CARP2 and CARP1 from soluble heart extracts of adult WT, MLP knockout or CARP1 knockout mice by using either CARP1 antibodies or normal rabbit IgG as control (visible in Ponceau stain).