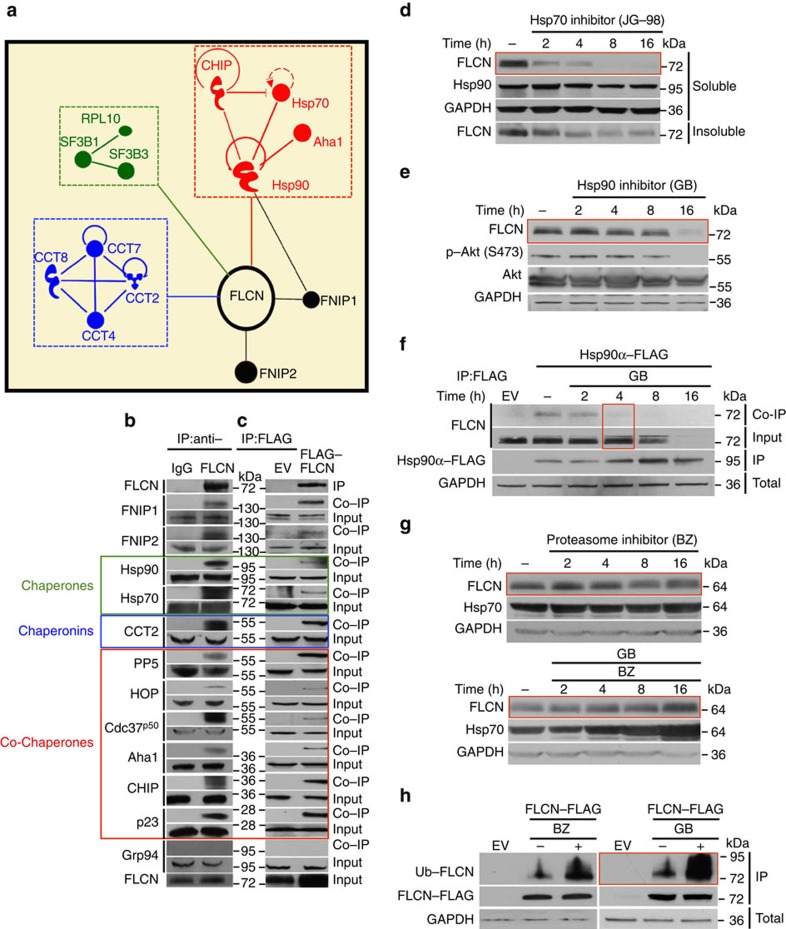
Figure 1. Folliculin is a new client of Hsp90.
(a) FLAG–FLCN was expressed and isolated from HEK293 cells. Profile of interacting proteins determined by MALDI–time of flight. Red nodes represent chaperones and co-chaperones, blue nodes are chaperonins and green nodes are splicing factors and ribosomal proteins. (b) FLCN was isolated from HEK293 cell lysates using anti-FLCN or IgG (control) and immunoblotted with indicated antibodies to confirm protein interactions. (c) HEK293 cells were transiently transfected with FLAG–FLCN or empty vector control (EV), immunoprecipitated and immunoblotted with indicated antibodies to confirm interacting proteins. (d) HEK293 cells were treated with 10 μM of the Hsp70 inhibitor JG-98 at the indicated time points. FLCN protein stability in soluble and insoluble fraction was assessed by immunoblotting. (e) HEK293 cells were treated with 1 μM GB at the indicated time points. FLCN protein stability was assessed by immunoblotting. Akt and Phospho-S473-Akt were used as positive controls. (f) Hsp90α–FLAG was transiently expressed in HEK293 cells. Cells were treated with 1 μM GB for the indicated times. Hsp90α–FLAG was immunoprecipitated and co-IP of FLCN was examined by immunoblotting. (g) HEK293 cells were treated with 50 nM of the proteasome inhibitor bortezomib (BZ) for the indicated times. FLCN protein levels were evaluated at the indicated time points by immunoblotting (upper blots). HEK293 cells were also treated with 1 μM GB for 1 h before addition of 50 nM BZ. Immunoblotting was used to evaluate the FLCN level for the indicated time points (lower blots). (h) Empty vector (EV) or FLAG–FLCN was used to transiently transfect HEK293 cells for 24 h then treated for 4 h with either 50 nm BZ or 1 μM GB. FLAG–FLCN was immunoprecipitated and ubiquitination was examined by immunoblotting with a pan-anti-ubiquitin antibody.