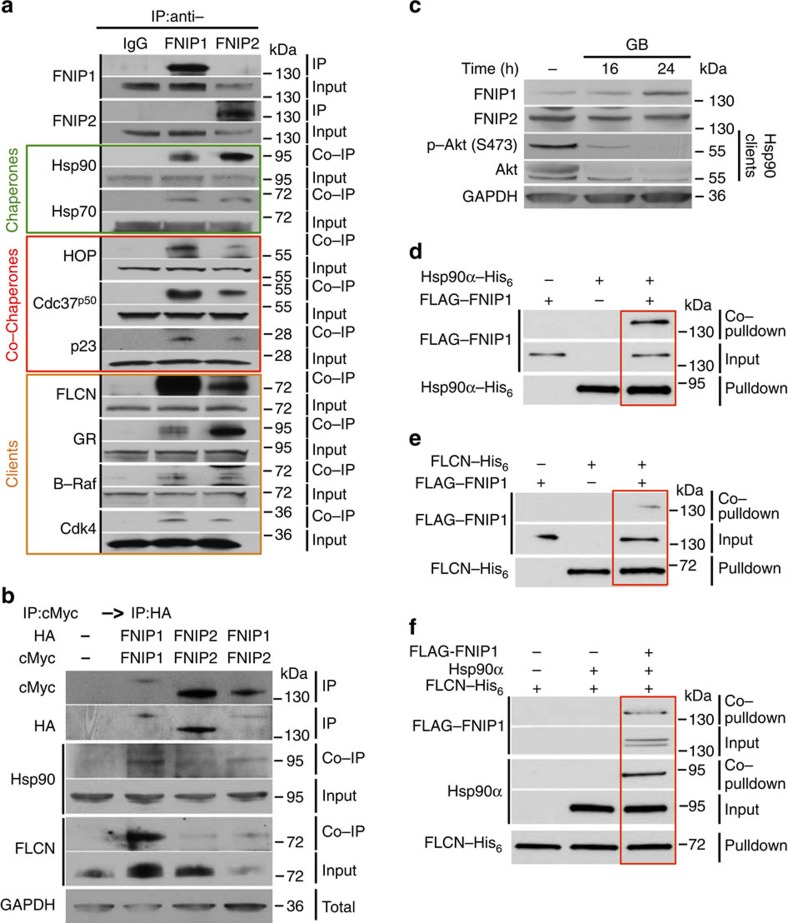
Figure 2. FNIPs facilitate FLCN binding to the Hsp90 chaperone.
(a) FNIP1 and FNIP2 were isolated from HEK293 cell lysates using anti-FNIP1, anti-FNIP2 or IgG (control) and immunoblotted with indicated antibodies to confirm protein interaction. (b) HA–FNIP1 and HA–FNIP2 were transiently co-expressed with either cMyc–FNIP1 or cMyc–FNIP2 in HEK293 cells. cMyc–FNIP1 and cMyc–FNIP2 were first isolated followed by IP of HA–FNIP1 and HA–FNIP2 from the same samples. The quality of HA–FNIP1:cMyc–FNIP1 and HA–FNIP2:cMyc–FNIP2 homodimers and HA–FNIP1:cMyc–FNIP2 heterodimer were assessed by immunoblotting. Co-IP of Hsp90 and FLCN was also assessed by western blotting. (c) HEK293 cells were treated with 1 μM GB for the indicated times, and FNIP1 and FNIP2 protein levels were determined by immunoblotting. Akt and Phospho-S473-Akt were used as positive controls. (d) FLAG–FNIP1 interacts with Hsp90α–His6 in vitro. Bacterially expressed and purified Hsp90α–His6 was bound to Ni-NTA agarose and then incubated with 10 ng pure FLAG–FNIP1. Hsp90α–His6 pulldown and FLAG–FNIP1 co-pulldown were assessed by immunoblotting. (e) FLAG–FNIP1 interacts with FLCN–His6 in vitro. Bacterially expressed and purified FLCN–His6 was bound to Ni-NTA agarose and then incubated with 10 ng pure FLAG–FNIP1. FLCN–His6 pulldown and FLAG–FNIP1 co-pulldown were assessed by immunoblotting. (f) FLCN–His6, FLAG–FNIP1 and Hsp90α tri-complex in vitro. Bacterially expressed and purified FLCN–His6 was bound to Ni-NTA agarose and followed by incubation with 10 ng FLAG–FNIP1 and then 10 ng untagged Hsp90α in vitro. FLCN–His6 pulldown, and FLAG–FNIP1 and Hsp90α co-pulldown were examined by immunoblotting.