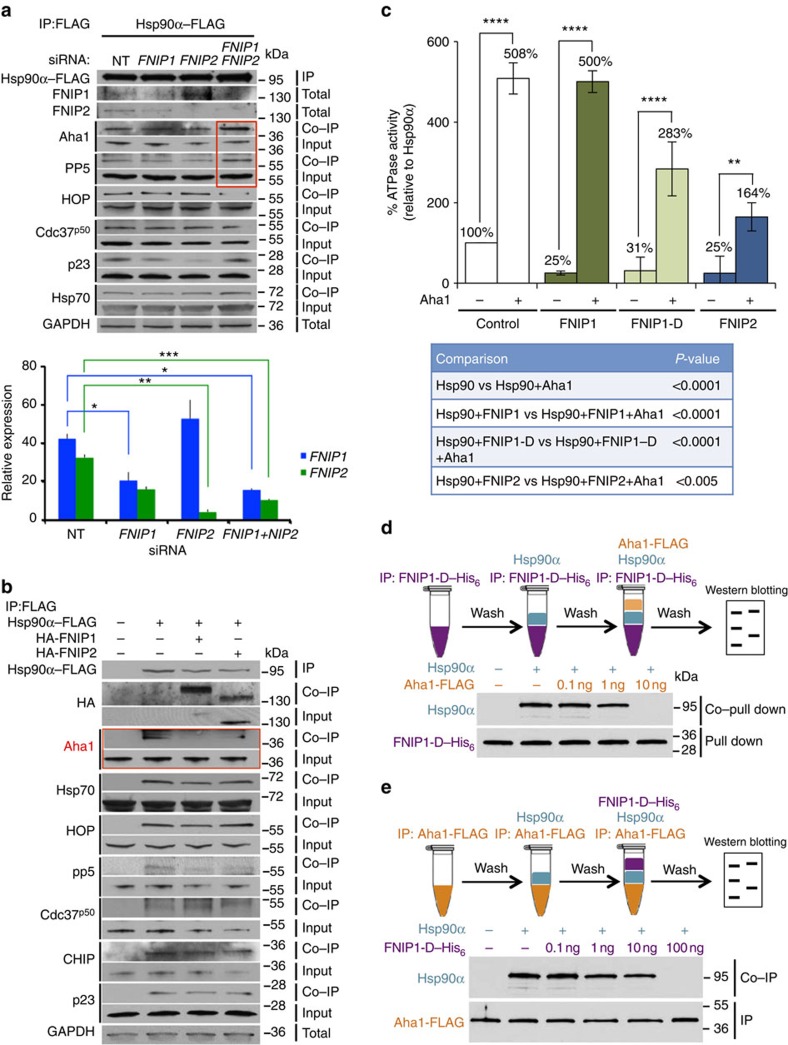
Figure 6. FNIPs compete with the Aha1 co-chaperone for binding to Hsp90.
(a) Hsp90α–FLAG was transiently expressed in HEK293 cells for 24 h followed by siRNA knockdown of FNIP1 and/or FNIP2. Hsp90α–FLAG was immunoprecipitated (IP) and co-IP of the co-chaperones was assessed by immunoblotting. Densitometry of the western blotting for FNIPs is represented as mean±s.d. A Student's t-test was performed to assess statistical significance (*P<0.05, **P<0.005 and ***P<0.0005). (b) HEK293 cells were transiently co-transfected with Hsp90α–FLAG and HA–FNIP1 or HA–FNIP2. Hsp90α–FLAG was isolated and co-IP of co-chaperones examined by immunoblotting. (c) HA–FNIP1, HA–FNIP1-D and HA–FNIP2 inhibited Hsp90α–HA ATPase activity after 30 min. Addition of 1.3 μM Aha1–FLAG stimulated the ATPase activity. All the data represent mean±s.d. A Student's t-test was performed to assess statistical significance (**P<0.005 and ****P<0.0001). (d) FNIP1 and Aha1 compete for binding to Hsp90α. FNIP1-D–His6 was attached to Ni-NTA agarose and then incubated with Hsp90α. Ni-NTA agarose was then washed and incubated with the indicated amounts of Aha1–FLAG. (e) Aha1–FLAG attached to anti-FLAG M2 affinity gel was incubated with Hsp90α initially and then washed and incubated with indicated amounts of the FNIP1-D–His6.