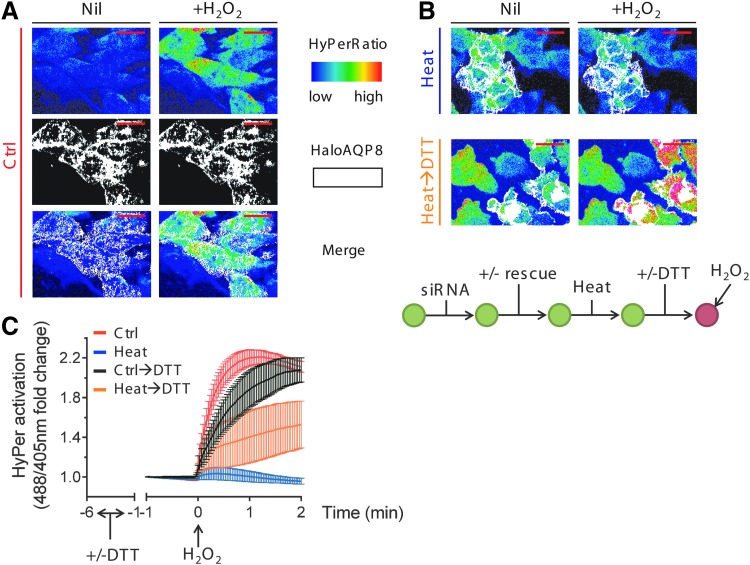
FIG. 2.
AQP8 is the target of redox-dependent regulation of H2O2 permeability. (A) Frames extracted from a representative video of ≥3 experiments, highlighting the kinetics of H2O2 import into silenced HeLa cells reconstituted with HaloAQP8 before (left panel) and after (right panel) addition of 50 μM exogenous H2O2. The 488/405 nm ratio is shown in pseudocolor (upper panel, the scale used is indicated in the insert). Cells positively transfected with HaloAQP8 wt are colored in white (middle panel). HyPerfluorescence ratio at baseline is variable from cell to cell, but the mean within a cell population is nearly the same in all experiments. The variability at the single-cell level explains why the basal colors presented in panels or figures sometimes differ. The scale bars correspond to 50 μm. Nil, nontreated cells. (B). The upper panels correspond to frames extracted from a video as in (A), showing no changes in the HyPerCyto ratios in HeLa cells reconstituted with HaloAQP8 under stress. The frames in lower panels show that recovery of H2O2 transport occurs only in cells expressing the transgene (colored in white) after a 5-min treatment with 5 mM DTT and two washes before the analysis. The experimental flow is depicted on the lower scheme for clarity. (C) Kinetics of H2O2 import into HeLa cells, in which AQP8 was silenced and then reconstituted with a silencing-resistant vector, driving the expression of HaloAQP8. Cells were heat shocked and then incubated with (orange trace) or without (blue trace) 5 mM DTT for 5 min. Results represent the mean fold changes of the 488/405 nm ratio measured by confocal laser scanning, plotted against time. Average of ≥3 experiments ± SEM. AQP8, aquaporin-8. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars