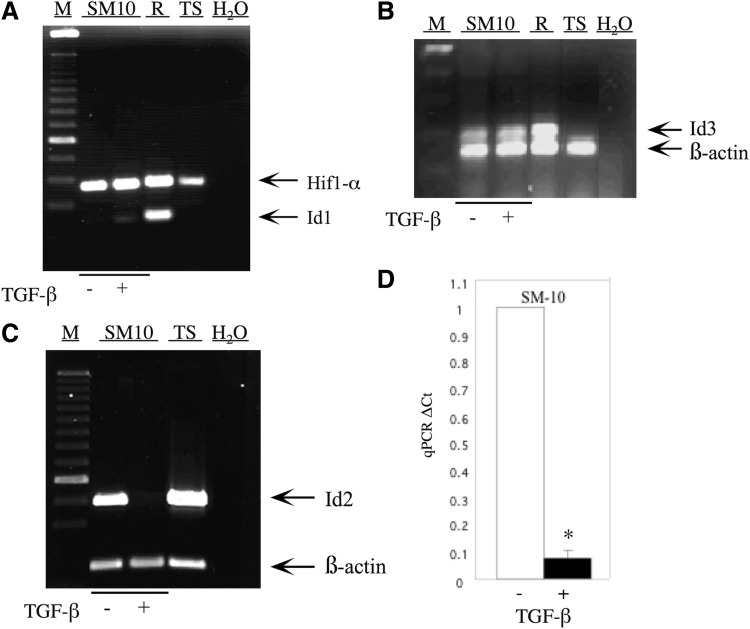
FIG. 1.
Id2 expression is inhibited on differentiation of the labyrinthine trophoblast progenitor cell line, SM10. Total RNA from TGF-β or vehicle-treated, SM10 cells was RT-PCR amplified with appropriate primers (Table 1) and resolved on a 1% agarose gel. Expression of Id1 (A), Id2 (C), and Id3 (B) in TGF-β or vehicle-treated SM10 cells is shown. Undifferentiated Rcho-1 (R) and TS cells (TS) were used as positive controls; 100 bp DNA marker (M) was utilized in estimating the sizes of PCR amplification products. Co-amplification of β-actin was used for semiquantitative normalization. Due to similarity in size, Hif1-α was co-amplified for semiquantitative normalization of Id1 samples (A). H2O was substituted for reverse-transcribed RNA as a control to indicate no genomic contamination (A–C). TGF-β-induced changes in Id2 expression were quantified by real-time qPCR (D). Expression of Id2 levels was determined by normalization to β-actin (endogenous control) in each sample by using the comparative CT method with the TaqMan MGB probe for qPCR with a fold change = 2−ΔΔCT method. Error bars represent standard deviation from the mean *P < 0.0005. RT-PCR, reverse transcription-polymerase chain reaction; qPCR, quantitative PCR; TS, trophoblast stem.