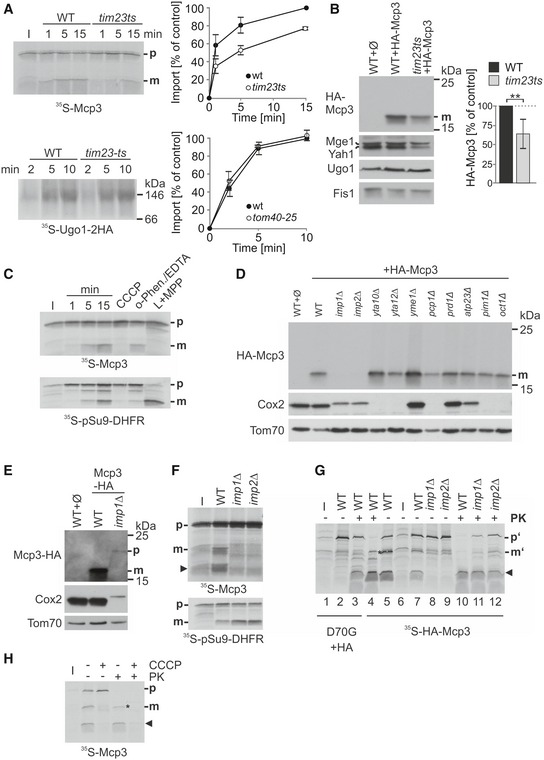
Import of Mcp3 is dependent on the TIM23 complex. Mitochondria isolated from wild‐type and
tim23ts cells were incubated with radiolabelled Mcp3 for the indicated time periods. Further treatment and analysis (
n = 3; SD) was as described for Fig
5D.
Steady‐state levels of HA‐Mcp3 are lower in cells harbouring a temperature‐sensitive TIM23 allele. Crude mitochondria were obtained at the non‐permissive temperature from WT or tim23ts cells containing a plasmid expressing HA‐Mcp3. Samples were analysed by SDS–PAGE and immunodecoration with antibodies against the HA‐tag, the matrix proteins Mge1 and Yah1 as typical TIM23 substrates, Ugo1 and Fis1 as TIM23‐independent substrates. HA‐Mcp3 levels were quantified in relation to Fis1 levels. Levels in WT cells were set to 100%. The bar diagram shows the mean with standard deviation of six independent experiments (n = 6; SD; **, P < 0.01; two‐tailed Student's t‐test).
Import and processing of Mcp3 is dependent on the mitochondrial membrane potential. Isolated mitochondria were incubated with radiolabelled Mcp3 or pSu9‐DHFR as control for the indicated time periods or for 15 min in the presence of either CCCP, or o‐phenanthroline and EDTA (o‐Phen./EDTA). After import, mitochondria were reisolated and analysed by SDS–PAGE and autoradiography. L + MPP: radiolabelled proteins were incubated with recombinant MPP.
Mcp3 is processed by Imp1/2. Single‐deletion strains of known mitochondrial proteases and peptidases were transformed with a plasmid expressing HA‐Mcp3. Crude mitochondria were isolated and analysed by SDS–PAGE and immunodecoration with antibodies against the indicated proteins and the HA‐epitope. Cox2, a known substrate of IMP; Tom70, a mitochondrial outer membrane protein.
C‐terminally tagged Mcp3‐HA is processed by Imp1. WT and imp1Δ cells were transformed with a plasmid expressing Mcp3‐HA, and further analysis was performed as described in (D).
Mcp3 is processed by Imp1/2 after
in vitro import into mitochondria. Mitochondria isolated from WT,
imp1Δ or
imp2Δ strains were incubated with radiolabelled precursor of Mcp3 or pSu9‐DHFR as control. Further treatment and analysis was as described in Fig
5A.
Full‐length Mcp3 precursor accumulates during import if IMP processing is abolished. Radiolabelled internally HA‐tagged Mcp3 without (HA‐Mcp3) or with mutation in the Imp1 cleavage site (D70G + HA) were incubated with mitochondria as in (F). After import, samples were incubated with or without PK (20 μg/ml). Samples were analysed by SDS–PAGE and autoradiography.
Mitochondrial membrane potential is required for efficient import of Mcp3 precursor into mitochondria. Isolated mitochondria were incubated with radiolabelled Mcp3 in the presence or absence of CCCP. After import, mitochondria were incubated with PK as in (G) and analysed by SDS–PAGE and autoradiography. I, 20% of radiolabelled precursor protein used in each import reaction; the arrowhead marks an additional band of the size of the cleaved N‐terminus of Mcp3.