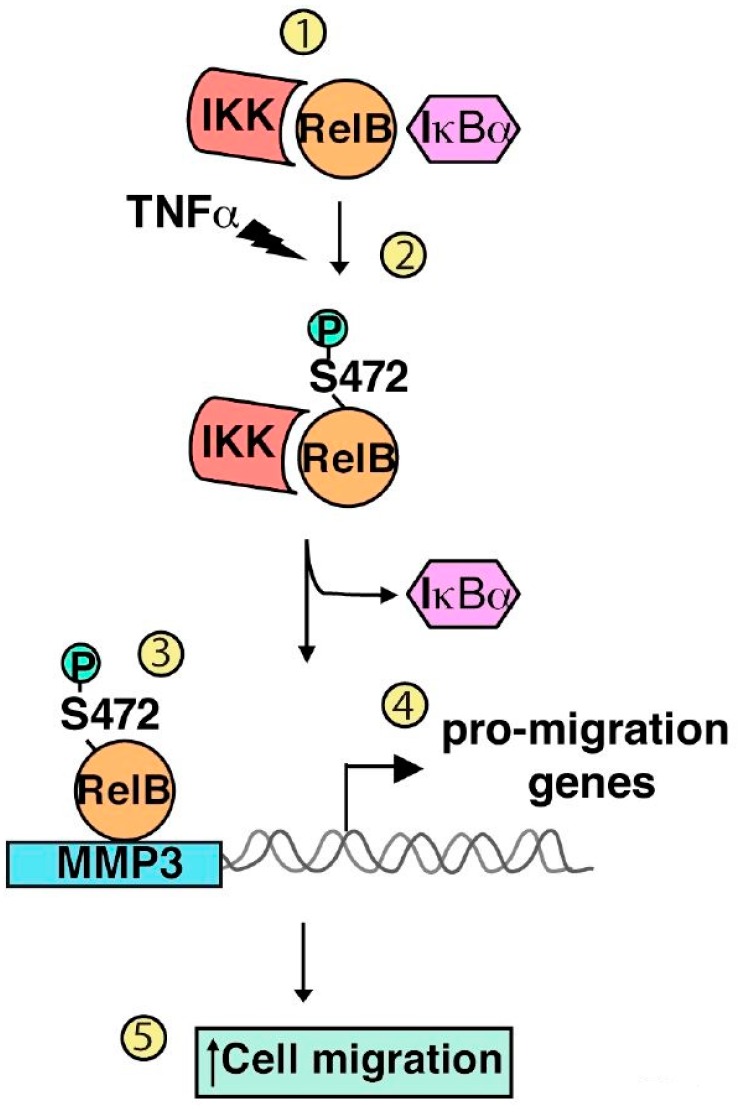
Figure 2.
Model for RelB serine-472 phosphorylation acting as an activator of inflammation-mediated cell migration. The IκB kinase (IKK) complex constitutively interacts with the RelB subunit of NF-κB [1]. Activation of IKK upon prolonged TNFα treatment (at least 6 hours) causes phosphorylation of RelB on serine 472 [2]. It allows nuclear ReB to dissociate from its interaction with the inhibitory protein IκBα and to bind to the promoter of pro-migration genes such as MMP3 [3], thereby resulting in selective NF-κB target gene expression involved in the control of TNFα-induced cell migration [4]. TNFα-induced IKK-driven ReB serine-472 phosphorylation is subsequently required for efficient cell migration in an MMP3-dependent manner [5].