Abstract
Mycobacteriosis is the second most common infectious disease in zebrafish research colonies, and most often this is caused by Mycobacterium chelonae. The infection is characterized by multiple granulomas in the kidney, coelomic cavity, particularly the ovary. However, most fish still appear clinically normal. Developmental genetics remain a primary area of research with the zebrafish model, and hence, an important use of adult zebrafish is as brood fish to produce embryos. We investigated the effects of experimentally induced M. chelonae infections on fecundity. A total of 480 5D wild-type zebrafish were divided into four groups: controls, males infected, females infected, and both sexes. Exposed fish developed high prevalence of infection, including many females with ovarian infections. Fish were then first subjected to four separate group spawns with four replicate tanks/group. Then, a third of the fish were subjected to pairwise spawns, representing 20 pairs/group, and then the pairs were evaluated by histopathology. Overall, the group and pairwise spawns resulted numerous eggs and viable embryos. However, we found no statistical correlations between infection status and number of eggs or viability. In contrast to Egg Associated Inflammation and Fibroplasia, lesions in infected ovaries were more localized, with large regions of the ovary appearing normal.
Introduction
Mycobacteriosis is the second most common infectious disease in zebrafish held in research facilities.1–4 Based on diagnostic submissions to the Zebrafish International Resource Center (ZIRC), the disease occurred in 32–48% of zebrafish laboratories between 2006 and 2014. The three most common species of Mycobacterium causing mycobacteriosis in zebrafish are Mycobacterium chelonae, Mycobacterium Marinum, and Mycobacterium hemophilum.4 The former two species are highly pathogenic to zebrafish, but M. chelonae is the most common,1–5 is less pathogenic than the other two species,6–8 and is often detected in apparently healthy fish.3,4,8 Many laboratories use adult fish solely for embryo production, and hence, the effects of chronic, often subclinical, infections on fecundity and embryogenesis would be a particular concern in this setting.9 Given the prevalence of M. chelonae infections in zebrafish, particularly ovarian infections, we investigated the effects of subclinical infection on fecundity and embryo survival.
Methods
Fish and husbandry
5D wild-type zebrafish were obtained from the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University. Fish were housed in a biosafety level 2 (BSL-2) room in a flow through system. Incoming municipal water source was filtered, dechlorinated, and heated to 27°C; fish were subjected to a 14/10-h light/dark photoperiod, pH 7.0–7.4, alkalinity 80 ppm, hardness 75 ppm, conductivity 135 μS. Fish were fed twice daily, except weekends, with the original commercial diet “master mix” used at the SARL.10
Mycobacteria exposure
When 7 months old, 15 male and 15 female fish were placed in 16 9L tanks, representing four groups with 4 tanks/group: (1) control, (2) infected males, (3) infected females, and (4) both males and females infected. Henceforth, the groups are designated Control, Male, Female, and Both. The appropriate fish were injected with 10 μL inoculum interperitoneally (IP) containing 1.7 × 104 M. chelonae H1E2 strain using 2-day-old culture following exposure methods of Watral and Kent.6 The H1E2 strain was selected; it was a common strain found in zebrafish at a large facility (3). As this was a lower dose than our previous studies, we injected the appropriate fish again 2 weeks later with 1 × 105 bacteria, resulting in a total exposure dose of 1.2 × 105. Fish designated as not exposed were injected with 10 μL sterile PBS. An additional 20 fish were injected at the same times and used as a guide to determine the infection status before conducting spawning experiments as described below.
To avoid egg retention, all tanks for fish were periodically spawned at the following time points: 0, 2, 6 weeks postinjection (PI, after first injection). At 1600 h, each tank of 30 fish was placed in a breeding cage with a screen bottom (dimensions) submerged in a 16 L tank containing 8 L of water and an air stone. Tanks were placed on a slant with air flow and slope adjusted so all tanks are as similar as possible. The breeding room received low light starting at 0600 the next day and then switched to bright light at 0730. At 8 am, all fish were returned to their tanks and eggs were collected.
Group spawning
At 10, 12, 14, and 16 weeks PI, all groups were spawned and data on total eggs, total viable eggs, and survival to hatch were obtained. Collection consisted of pouring spawn water from tanks through a sieve and rinsing them with sterile system water into containers. The embryos were sorted by first counting the number of nonviable eggs and then placing 300 viable embryos from each tank into three 150 mm diameter glass Petri dishes, with 100 embryos/100 mL embryo media and incubated in a single layer at 27°C. All remaining embryos from each group were fixed in 10% neutral buffered formalin. After 2 h when the yolk became opaque, the fixed embryos were placed in a Petri dish and photographed for future counting. Everyday for 5 days postfertilization (DPF) the 300 embryos were screened for mortality.
Pairwise spawning
Five pairs of fish from each of the 16 tanks were evaluated for the same fecundity endpoints as the group spawn testing. At 2 weeks after the final group spawn, the one third of the fish from each tank were spawned in pairs. All 5 pairs from each of one group tank were set up in various paired spawning cages, and 20 pairs/day were spawned over 4 days in either of the two spawning cage types: type A = Thoren Caging Systems (Hazelton, PA) 10 × 11 × 17 cm; type B was Aquaneering type (Aquaneering, Inc., San Diego, CA) 8.5 × 7.5 × 19 cm. While there was some variability in the spawning cage type, each group and tanks within had the same combination of cage types (Table 1). Moreover, each cage was treated as similarly as possible (500 mL water/each, same slope and water depth, and so on) under the same conditions as the group spawns with the exception of air stones. After spawning, each pair was euthanized, weighed, and preserved for histological evaluation. Fecundity endpoints were the same as the group spawn, except embryo mortality was only evaluated at 1 and 5 dpf.
Table 1.
Effects of Mycobacterium chelonae on Fecundity and Percent Survival to Hatch in Four Groups of 5D Zebrafish in Pairwise Spawning; Controls, Infected Males, Infected Females, and Both Sexes Infected
| Histology: Myco | |||||||
|---|---|---|---|---|---|---|---|
| Tank number | Pair/tank type | Eggs No/% viable/% survival | Body | Ovary | Testis | Weight (mg) female/male | |
| Control | |||||||
| 1 | 1/A | 0/NA/NA | 0 | 0 | 0 | 242/343 | |
| 1 | 2/A | 0/NA/NA 0 | 0 | 0 | 0 | 482/437 | |
| 1 | 3/B | 79/100/89.9 | 0 | 0 | 0 | 351/295 | |
| 1 | 4/B | 177/99.4/98.3 | 0 | 0 | 0 | 346/345 | |
| 1 | 5/B | 479/99.8/89 | 0 | 0 | 0 | 488/318 | |
| Average | 147/99.7/92.4 | 0 | 0 | 0 | |||
| 2 | 1/A | 364/100/99.7 | 0 | 0 | 0 | 377/309 | |
| 2 | 2/A | 375/100/.33 | 0 | 0 | 0 | 287/319 | |
| 2 | 3/B | 0/NA/NA | 0 | 0 | 0 | 341/383 | |
| 2 | 4/B | 124/100/68.6 | 0 | 0 | 0 | 330/317 | |
| 2 | 5/B | 0/NA/NA | 0 | 0 | 0 | 393/290 | |
| Average | 172.6/100/56.2 | 0 | 0 | 0 | |||
| 3 | 1/A | 195/99.5/78.4 | 0 | 0 | 0 | 437/399 | |
| 3 | 2/A | 171/100/97.1 | 0 | 0 | 0 | 328/313 | |
| 3 | 3/B | 0/NA/NA | 0 | 0 | 0 | 340/375 | |
| 3 | 4/B | 486/99.4/48.3 | 0 | 0 | 0 | 400/382 | |
| 3 | 5/B | 0/NA/NA | 0 | 0 | 0 | 492/240 | |
| Average | 170.4/99.6/74.6 | 0 | 0 | 0 | |||
| 4 | 1/A | 330/100/65.5 | 0 | 0 | 0 | 422/371 | |
| 4 | 2/A | 0/NA/NA | 0 | 0 | 0 | 392/322 | |
| 4 | 3/B | 132/100/98.5 | 0 | 0 | 0 | 308/293 | |
| 4 | 4/B | 232/100/72.0 | 0 | 0 | 0 | 382/247 | |
| 4 | 5/B | 321/100/95.7 | 0 | 0 | 0 | 374/341 | |
| Average | 203/100/82.9 | 0 | 0 | 0 | |||
| Male | 1 | 1/A | 0/NA/NA | 0/3 | 0 | 0 | 566/262 |
| 1 | 2/A | 407/100/99.7 | 0/2 | 0 | 0 | 510/267 | |
| 1 | 3/B | 100/100/98 | 0/3 | 0 | 0 | 265/297 | |
| 1 | 4/B | 0/NA/NA | 0/2 | 0 | 0 | 452/335 | |
| 1 | 5/B | 216/216/69.0 | 0/1 | 0 | 0 | 448/363 | |
| Average | 144.6/100/88.9 | ||||||
| 2 | 1/A | 112/100/68.75 | 3/1 | 3 | 0 | 408/310 | |
| 2 | 2/A | 424/99.5/89.67 | 2/1 | 2 | 0 | 371/441 | |
| 2 | 3/B | 131/100/71.76 | 0/2 | 0 | 0 | 324/289 | |
| 2 | 4/B | 43/100/95.35 | 0/2 | 0 | 0 | 339/402 | |
| 2 | 5/B | 96/100/96.88 | 0/3 | 0 | 0 | 327/284 | |
| Average | 161.2/99.9/84.5 | ||||||
| 3 | 1/A | 242/99.6/96.3 | 0/1 | 0 | 0 | 433/443 | |
| 3 | 2/A | 378/95.5/97.7 | 0/2 | 0 | 1 | 442/407 | |
| 3 | 3/B | 0/NA/NA | 0/0 | 0 | 1 | 470/368 | |
| 3 | 4/B | 274/99.3/99.6 | 0/2 | 0 | 0 | 375/384 | |
| 3 | 5/A | 45/97.8/97.7 | 0/0 | 0 | 0 | 332/442 | |
| Average | 187.8/98.1/97.8 | ||||||
| 4 | 1/A | 557/100/95.5 | 0/2 | 0 | 0 | 396/373 | |
| 4 | 2/A | 623/99.4/90 | 0/0 | 0 | 0 | 466/402 | |
| 4 | 3/B | 178/98.9/98.9 | 0/3 | 0 | 3 | 344/342 | |
| 4 | 4/B | 147/100/54.4 | 0/3 | 0 | 0 | 273/334 | |
| 4 | 5/B | 318/100/97.5 | 0/2 | 0 | 0 | 350/317 | |
| Average | 364.6/99.7/87.3 | ||||||
| Female | 1 | 1/A | 0/NA/NA | 1/0 | 0 | 0 | 461/318 |
| 1 | 2/A | 585/99.7/91 | 0/0 | 0 | 0 | 482/376 | |
| 1 | 3/B | 109/100/94.5 | 3/0 | 0 | 0 | 612/419 | |
| 1 | 4/B | 0/NA/NA | 0/0 | 0 | 0 | 879/390 | |
| 1 | 5/B | 318/100 | 2/0 | 0 | 0 | 393/382 | |
| 1 | Average | 202/99.1/84.2 | |||||
| 2 | 1/A | 127/100/79.5 | 2/0 | 0 | 0 | 420/352 | |
| 2 | 2/A | 209/100/52.6 | 0/0 | 3 | 0 | 402/387 | |
| 2 | 3/B | 182/100/98.9 | 0/0 | 0 | 0 | 431/353 | |
| 2 | 4/B | 151/99.3/91.3 | 0/0 | 2 | 0 | 334/363 | |
| 2 | 5/B | 0/NA/NA | 1/0 | 0 | 0 | 457/403 | |
| Average | 133.8/99.8/81.0 | ||||||
| 3 | 1/A | 0/NA/NA | 0/0 | 0 | 0 | 743/320 | |
| 3 | 2/A | 598/100/30.3 | 2/0 | 2 | 0 | 464/374 | |
| 3 | 3/B | 505/99.8/96 | 0/0 | 1 | 0 | 447/318 | |
| 3 | 4/B | 248/99.6/41.7 | 0/0 | 2 | 0 | 406/305 | |
| 3 | 5/B | 61/98.4/98.3 | 0/0 | 0 | 0 | 354/301 | |
| Average | 282.4/99.5/66.59 | ||||||
| 4 | 1/A | 0/NA/NA | 0/0 | 1 | 0 | 411/416 | |
| 4 | 2/A | 319/100/98.8 | 0/0 | 1 | 0 | 452/330 | |
| 4 | 3/B | 0/NA/NA | 3/0 | 1 | 0 | 439/405 | |
| 4 | 4/B | 381/100/97.7 | 0/1 | 1 | 0 | 497/.435 | |
| 4 | 5/B | 171/100/69.6 | 0/0 | 2 | 0 | 336/333 | |
| Average | 174.2/100/88.7 | ||||||
| Both | 1 | 1/A | 222/99.5/88.7 | 1/2 | 0 | 2 | 456/274 |
| 1 | 2/A | 399/100/100 | 0/1 | 0 | 0 | 343/227 | |
| 1 | 3/B | 153/100/94.8 | 2/3 | 2 | 2 | 341/322 | |
| 1 | 4/B | 218/100/98.6 | 0/3 | 2 | 2 | 419/413 | |
| 1 | 5/B | 107/100/98.1 | 2/3 | 2 | 1 | 468/352 | |
| Average | 219.8/99.9/96.0 | ||||||
| 2 | 1/A | 503/99.8/41.3 | 1/2 | 0 | 1 | 427/342 | |
| 2 | 2/A | 357/96.1/95.0 | 1/2 | 1 | 0 | 377/350 | |
| 2 | 3/B | 80/100/96.3 | 0/0 | 0 | 0 | 500/.219 | |
| 2 | 4/B | 323/100/55.3 | 0/1 | 1 | 0 | 341/.303 | |
| 2 | 5/B | 112/100/92.9 | 1/1 | 0 | 0 | 426/.388 | |
| Average | 275/99.2/762 | ||||||
| 3 | 1/A | 479/99.6/88.0 | 0/3 | 1 | 0 | 399/371 | |
| 3 | 2/A | 318/100/98.7 | 0/1 | 2 | 0 | 365/321 | |
| 3 | 3/B | 488/97.9/92.3 | 0/3 | 0 | 3 | 403/372 | |
| 3 | 4/B | 328/100/67.7 | 0/2 | 0 | 0 | 411/377 | |
| 3 | 5/B | 237/97.5/72.3 | 2/2 | 2 | 0 | 390/422 | |
| Average | 370/99.0/83.8 | ||||||
| 4 | 1/A | 0/NA/NA | 2/2 | 2 | 0 | 315/288 | |
| 4 | 2/A | 584/100/98.3 | 3/3 | 3 | 0 | 508/323 | |
| 4 | 3/B | 147/98.6/93.1 | 1/3 | 0 | 0 | 333/347 | |
| 4 | 4/B | 525/99.8/92.7 | 0/3 | 3 | 0 | 400/436 | |
| 4 | 5/B | 198/79.8/93.0 | 1/2 | 0 | 0 | 528/316 | |
| Average | 290.8/94.55/87.0 | ||||||
Severity of lesions are scored 0–3 for either the entire body exclusive of the gonads (body) or for gonads.
Histology
Fish were preserved in Dietrich's solution, processed, and slides stained with Kinyoun's acid-fast as described by Peterson et al.11 Additional slides from selected fish were stained with hematoxylin and eosin to better illustrate lesion morphology. Severity of overall and gonad severity was scored by counting total numbers of granulomas containing acid fast bacteria in the coelomic cavity, ovaries, or testis. Score of 1 was 1–2 granulomas, 2 = multiple granulomas observed, 3 = prominent infections with granulomatous lesions occupying a large amount of the coelom or gonad. In addition, gondal development was assessed in as score as follows. Ovary, mature, with numerous eggs in all stages of development from immature oocytes to mature, vitellogenic eggs, or immature (artic eggs or lack of mature, vitellogenic eggs). Testis, mature (with spermatogonia through at least spermatids present versus immature or atrophied.
Statistics
Differences in fecundity endpoints for each sampling week between groups, pairs, or tanks were tested using ANOVA, where significant differences were identified by using a p-value threshold of p < 0.05 after correcting for multiple tests using a Bonferroni test. Group and pairwise spawn fecundity endpoints were also correlated with covariates (e.g., histological and weight data) using spearman's tests, which were similarly corrected using Bonferroni tests. Linear regression was used to model the interaction between fecundity endpoints and exposure group, tank type, weight, or histological covariates (i.e., histology total, histology gonads for both males and females).
Results
Group spawns
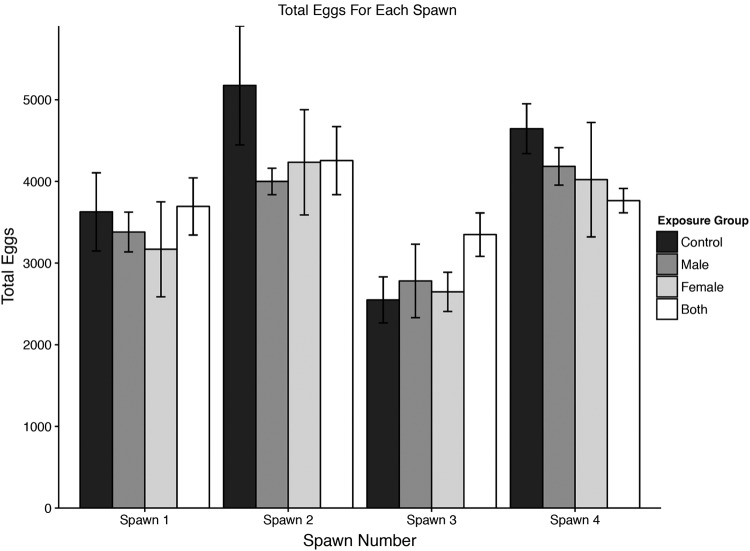
Examination of six fish from the M. chelonae-injected sentinel population at 8 week postinjection exhibited 100% incidence of infection. Therefore, we moved forward with group spawning trials. A total of 480 fish (equal males and females) from the four groups were spawned four times over a 6-week period starting at 10 weeks PI. Most spawns produced numerous eggs, ranging from about 2000 to over 6000 eggs/spawn, with a mean of 3800 eggs/spawn (Fig. 1). Most eggs showed high viability immediately after spawning, with only two spawns <94%. Survival to hatch was also good; two showed 86% and 88% survival and the remaining 62 spawns were all >90%. There was no detectable statistical difference between groups with the different infection parameters regarding total number of eggs, viable eggs at spawning, and survival to hatching. For example, the total eggs produced in the Control group was 63,338 compared to 60,244 for the group in which both males and females were infected (Fig. 1).
FIG. 1.
Group spawn. Total eggs counted in group spawns. Spawning was done four separate times for each of the four treatment groups; Control, Males = Infected Males, Females = Infected Females, Both = Both sexes infected. Fish released fewer eggs at spawn 3.
There was some variability in spawning success among the 4 spawning events for each group (Spawns 1–4), with Spawn 3 being the lowest for all three groups (Fig. 1). Using analysis of variance found that there is a significant difference in the total number of eggs produced across weeks (p < 0.05), and a Tukey's post hoc test determined that Spawn 3 produced significantly lower numbers of eggs across all groups compared to Spawns 2 and 4 (p < 0.05, Spawn 3 vs. Spawn 1 p = 0.15), and that Spawn 1 had a significantly lower number of eggs than Spawn 2 (p < 0.05, Spawn 1 vs. Spawn 4 p = 0.11).
Pair spawns
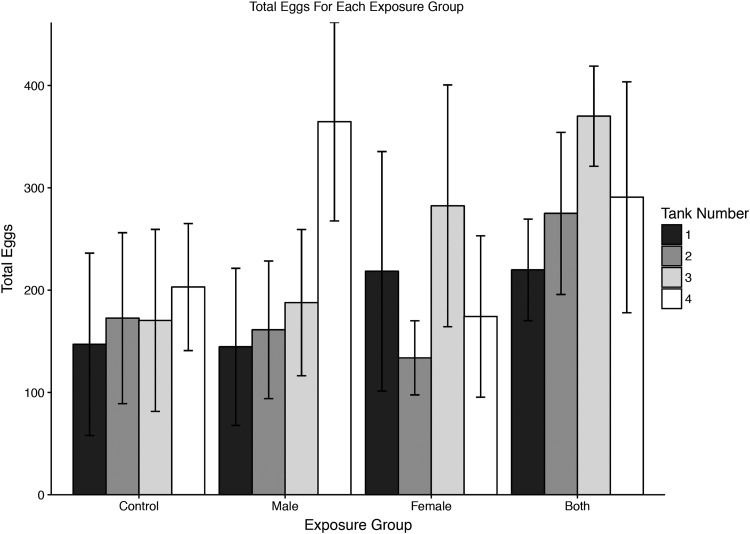
Spawning data
At 2 weeks after the last group spawn (18 weeks PEI), 20 pairs from each of the four groups were spawned in pairs. These represented about one third of the fish from each group, with pairs selected evenly from each of the three replicate tanks within each group (Fig. 2 and Table 1). Spawn success was more variable than with group spawns, ranging from 0 to 623 eggs. Eggs were >94% viable in all but two of the spawns. As with the group spawns, infected groups did not produce fewer eggs. In fact, 6 of the 20 pairs in the control group produced no eggs. Survival to hatching was quite variable, ranging from 33% to 98%, but was not correlated with the infection status. Indeed, two of the lowest survival spawns (33% and 48%) occurred within the control group.
FIG. 2.
Total eggs from pairwise spawns for four treatment groups (Control, Infected Males, Infected Females, Both sexes infected). Each tank represented four individual pairs. Tank numbers correspond with tank numbers in Table 1.
Histology
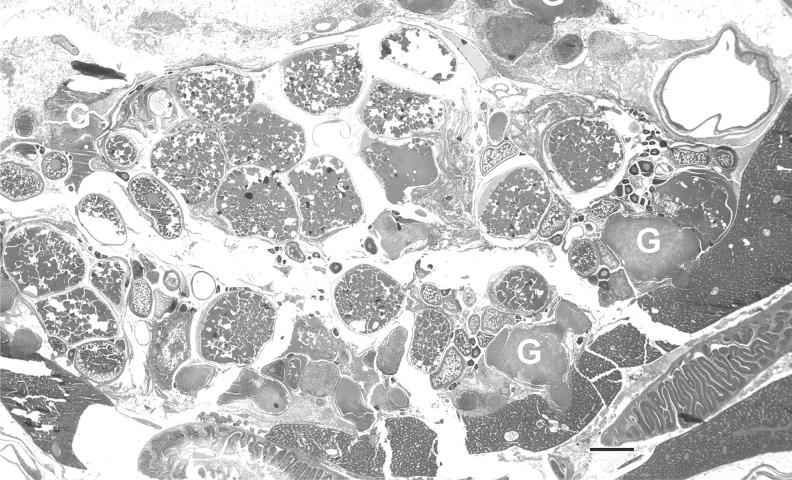
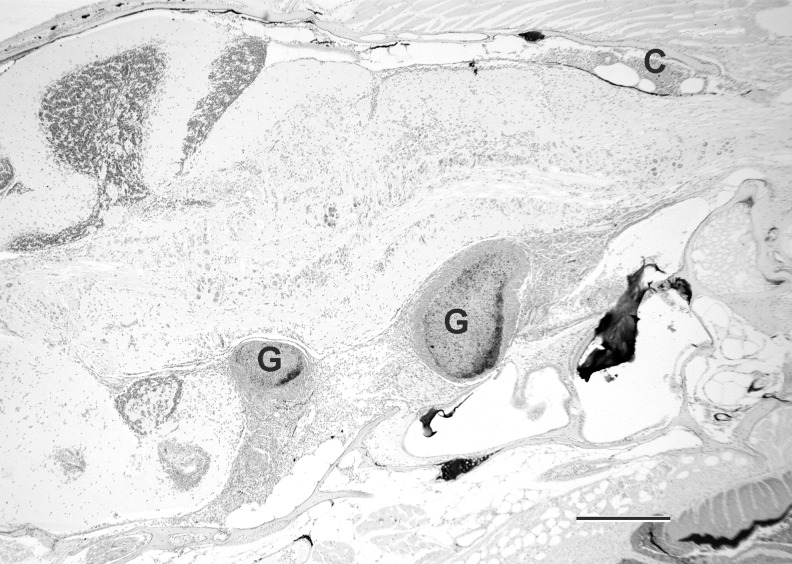
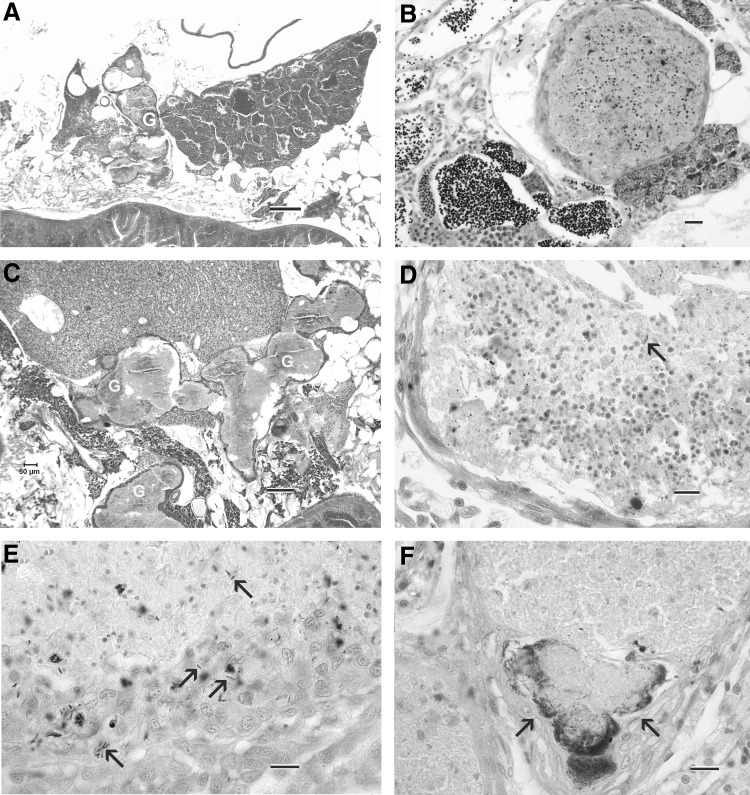
Histological evaluation performed on individual fish from each group showed an overall 86% prevalence of infection in the exposed fish (Table 1). Typical of mycobacteriosis, the infections were characterized by granulomas containing acid fast bacteria (Figs. 3–5). Most of the granulomas comprised a very thin layer of epithelioid cells, with large centers replete with macrophages and lacking caseous necrosis. Lesions outside of the gonads included granulomas in the mesenteries, kidney, liver, heart, muscle. One female fish in the Female group (Tank 4, fish 3) (Table 1) exhibited an extensive infection of the cranial cavity (Fig. 4). All fish, regardless of infection status, showed essentially normal gonadal development based on histology.
FIG. 3.
Female zebrafish (0.5 g) with Mycobacterium chelonae infection. hematoxylin and eosin (H&E). Bar = 300 μm. Numerous, coalescing granulomas (G) in the ovary and kidney region. This fish releases 112 eggs, and ovary is replete with vitellogenic eggs.
FIG. 4.
Female zebrafish with Mycobacterium chelonae infection of meningitis around brain. Granulomas (G) deep to the reticular formation in the brainstem and chronic inflammation (C) dorsal to medulla oblongata. Acid fast. Bar = 300 μm.
FIG. 5.
Histological sections of zebrafish with experimental Mycobacterium chelonae infections. (A) Testis with multiple cavernous granulomas. Note remaining testes exhibit normal stages of development. This pair spawned, producing numerous eggs that survived to hatching (Both group (Tank 1 fish 1). H&E. Bar = 100 μm. (B) High magnification of granuloma adjacent to testis, showing a degenerate center composed of necrotic debris surrounded by a thin layer of epithelioid macrophages. H&E. Bar = 20 μm. (C) Multiple coalescing granulomas (G) in coelomic cavity adjacent to liver. H&E. Bar = 100 μm. (D) Granuloma replete with plump foamy macrophages surrounded by a thick layer of flattened epithelioid macrophages. Few bacteria (arrows) were observed. Acid fast. Bar = 10 μm. Granuloma with numerous acid-fast bacilli at edge. Acid fast. Bar = 10 μm. (E, F) Numerous acid bacteria at the edges of a granulomas from liver (E) and ovary (F). Acid fast. Bar = 10 μm
About half of the females exhibited gonadal infections (Table 1 and Fig. 3). Ovarian infections varied from one or two focal granulomas (score 1) to extensive (score 3). However, even fish with extensive lesions exhibited large regions of the ovary lacking inflammatory changes and replete with eggs in all stages of development (Fig. 3). Three and six males in the Male and Both groups, respectively, had testicular infections. One male in the Male group had an extensive lymphosarcoma involving the thymus, gills, and epithelium of the gill chamber, respectively. Overall, gonadal infection severity was predictive of an individual's total infection severity as indicated by a spearmen's test of correlation among these parameters across all individuals subject to paired spawns (female: p = 0.046, rho = 0.356; male: p = 0.0094, rho = 0.397).
Incorrect infections
Mycobacteriosis was not observed in two males from the Male group and one male in the Both group. The infection was not detected in five and three females in the Female and Both groups, respectively. Conversely, the infection was observed in two females that were not deliberately infected in the Male group, and one of these fish showed both extensive ovarian and visceral infections.
Correlations of histology and fecundity
Several of the females that produced large numbers of eggs and a high prevalence of viable eggs and hatching had ovarian infections, while some pairs that failed to spawn were not infected, including pairs within both the controls and injected groups. One of the females with the most severe kidney, coelomic, and ovarian infections laid 112 eggs, in which most were all were viable and about 70% of the eggs hatched. In contrast, the one female with the severe cranial infection did not spawn.
An average of 252 eggs were produced by the females with gonadal infections, and an average of 281 eggs were produced from the pairwise spawns with the males with testicular infections. These results were similar to eggs produced by the control group when pairs that failed to spawn were removed from the analysis of that (256 eggs). Comparison of severity of total body mycobacteriosis and ovarian infections showed no statistical correlations with any of the fecundity endpoints. Indeed, even the uncorrected p-values were nonsignificant across the following comparisons, for whole body and ovarian infection status, respectively: total eggs at spawning (p = 0.89, p = 0.06 [corrected p = 1.0]), percent intact eggs (p = 0.41, p = 0.57), percent of embryos surviving to hatching (p = 0.15, p = 0.42) (Table 1). It is notable that the total eggs at spawning had a marginally significant raw p-value with the severity of ovarian infection, but the observed difference is likely due to random sampling given that the p-value erodes when permissive multiple test correction methods (i.e., q value p = 0.22) are applied. In fact, only 65% percent of the fish from the group of uninfected fish laid eggs, while 89% of the pairs in which at least one fish was infected spawned. Moreover, these analyses were repeated by removing the pairs of fish from the three exposed groups that did not show the predicted infections by histology, and the statistical results were qualitatively consistent with the aforementioned results.
Fish size
Comparisons of fish weight with fecundity and infection status showed no statistical correlations, and control uninfected fish were no larger than infected groups (Table 1). There was a potentially weak anticorrelation between the weight of male fish and egg viability (uncorrected p = 0.0066, rho = −0.34), although this is eroded after correcting for multiple tests using a Bonferroni correction. A more permissive correction method (i.e., q-value p = 0.043) indicates that this relationship may be meaningful, but this approach is prone to higher levels of false discovery.
Discussion
Group and pairwise spawn results were similar to previous studies in regard to overall egg numbers and survival to hatch, even in M. chelonae-exposed populations. For example, Spence and Smith12 (DATE) reported 169–185 eggs/clutch, whereas our study had an overall average of 280/pairwise spawn. Castranova et al.13 conducted a large fecundity experiment involving eight separate laboratories and some 200 pairwise spawns. In this study they reported about 130–140 eggs, with considerable variability, ranging from zero to over 1000 eggs/clutch. Likewise, survival of embryos in our study was similar, with the Castranova et al.13 having a mean survival from pairwise spawn at about 80% and a range of about 70–90%. There was no correlation between fish size and spawning success in our study. Indeed, some of the heaviest females did not spawn. Fish were weighed after spawning and perhaps the retained females in these females accounted for their larger weight.
Wild zebrafish in India and Bangladesh occur in a wide range of environments regarding temperature, conductivity, and pH.14 In research facilities, zebrafish are generally maintained and bred in recirculating systems, in which the pH hardness is close controlled. In the study by Castranova et al.13, conductivity among the 8 facilities was 550–2300 μs. Our laboratory maintains zebrafish in a flow-through system in which there is much lower conductivity. This is much lower than what is generally recommended for zebrafish,15 but still compared to other studies fecundity and egg survival in the present study would be considered acceptable.
Ramsay et al.16 achieved 60% infection with 5 × 104 in fish held under normal conditions. We obtained similar results, as we injected fish with M. chelonae at a higher dose (1.2 × 105) and observed 95% infection in the 80 of the fish that were examined by histology. In another study,11 we injected zebrafish with the same strain of M. chelonae at 1 × 106, resulting in 100% infection yet no mortality. One of the 80 fish that were examined that were not injected with M. chelonae exhibited prominent mycobacteriosis, which is consistent with the low level background infection we observed in fish from the SARL.17 We have since verified that the background infections at the SARL are caused by M. chelonae (unpublished data).
Pairwise spawns, with 20 pairs/group based on infection designation, did not exhibit significant differences with any of our fecundity endpoints. There was considerable intragroup variation in spawn success, with many pairs producing no eggs. This included fish from the control group as well as uninfected fish within the exposed groups. Mycobacterium chelonae often infects the ovaries and is associated with chronic oophoritis3. However, many of the pairs with prominent ovarian lesions still spawned successfully. Chronic inflammation of the ovary, regardless if it is caused by M. chelonae, has been associated with reduced fecundity. Rossteuscher et al.18 described an association between inflammation and egg atresia, but pointed out that it was unclear, which was the causative factor—that is, inflammation causing egg atresia versus degenerating eggs resulting in an inflammatory reaction. Nevertheless, this lesion, referred to as Egg Associated Inflammation and Hyperplasia (EAIF) may be linked to egg retention detection due to improper spawning schedules or other unknown underlying causes.17 However, we found the granulomatous inflammation associated with M. chelonae infections; it was much more focal and less extensive than the chronic inflammation observed with EAIF.
Mycobacterial infections often target the ovary,1,3 and these infection are often found in fish with extensive chronic inflammation. These would lead to the conclusion that the bacteria were the cause of the lesions, such as EAIF. In contrast, our results here suggest a possible alternative—that is, as mycobacteria thrive within macrophages, it is possible that this predilection for the ovaries by the bacteria is due to the abundance of macrophages typically found within ovaries associated with degenerating eggs, rather than the mycobacteria being the primary cause of the inflammation in many cases. Infected macrophages from other organs may be recruited to the ovary. We concluded our experiments after 18 weeks of exposure to M. chelonae, and hence, the possibility that much longer standing infections could lead to more extensive inflammation, as seen in with EAIF, cannot be excluded.
Some fish exhibited rather extensive infections in other organs. Interestingly, one female (C4-3) with severe menixite infection did not produce eggs, but with only one fish we cannot indicate a casual relationship. Note that we use the term menixites instead of meninges as cyprinids do not have “meninges” as in reptiles, birds, and mammals.19,20 Mammals have a dura mater and a pair of fused membranes termed the “leptomeninges” composed of the arachnoid mater and the pia mater. The pia mater directly adheres to blood vessels deep into the neuropil and so most inflammation in mammals involving the meninges is perivascular since the inflammation is at least temporarily held in place by the pia mater. In cyprinids, there does not appear to be a distinct pia and arachnoid mater.19,20 Therefore, the general term “meninx” is used to define the membranes surrounding the central nervous system.
In conclusion, we found no direct links between M. chelonae infections and fecundity. This is positive information for the zebrafish community in which adult fish are only used for breeding, given the prevalence of this bacterium in zebrafish research facilities. These negative findings on this particular research endpoint do not indicate that other laboratories using adult zebrafish as their research endpoints (e.g., studies on chronic diseases, inflammation, oncology) should ignore these underlying infections.9 In addition, the large variation in the number of eggs produced by at different times within the control group indicates that this particular fecundity endpoint may be so highly variable that any effect of infection is overwhelmed and not detectable. Regardless, we found no interaction between infection and the less variable fecundity endpoints of egg viability and the frequency of egg hatches. This study was conducted with a robust outbred line of fish that were spawned frequently, and thus, the results may not apply to less fecund and fastidious lines of zebrafish.
Acknowledgments
This study was funded, in part, by NIH ORIP 2R24OD010998-11 to M. Kent, and fish were provided by the Sinhubber Aquatic Resource Center. Enivronmental Health Sciences Center. NIH NIEHS P30 ES000210.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, et al. . Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem Physiol Part C 2004;138:383–390 [DOI] [PubMed] [Google Scholar]
- 2.Murray KN, Bauer J, Tallen A, Matthews JL, Westerfield M, Varga ZM. Characterization and management of asymptomatic Mycobacterium infections at the Zebrafish International Resource Center. J Am Assoc Lab Anim Sci 2011;50:675–679 [PMC free article] [PubMed] [Google Scholar]
- 3.Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Dis Aquatic Org 2008;82:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in zebrafish colonies. ILAR J 2012;53:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp Med 2000;50:666–672 [PubMed] [Google Scholar]
- 6.Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comp Biochem Physiol Part C 2007;145:55–60 [DOI] [PubMed] [Google Scholar]
- 7.Ostland VE, Watral V, Whipps CM, Austin FW, St-Hilaire S, Westerman ME,et al. . Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass (Morone chrysops x Morone saxatilis) and zebrafish (Danio rerio). Dis Aquat Org 2008;79:107–118 [DOI] [PubMed] [Google Scholar]
- 8.Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol Lett 2007;270:21–26 [DOI] [PubMed] [Google Scholar]
- 9.Kent ML, Harper C, Wolf JC. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR J 2012;53:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller GW, Truong L, Barton CL, Labut EM, Bebold KM, Traber MG, et al. . The influences of parental diet and vitamin E intake on the embryonic zebrafish transcriptome. Comp Biochem Physiol D Genom Proteom 2014;10:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson TS, Kent ML, Ferguson JA, Watral VG, Whipps CM. Comparison of fixatives and fixation time on PCR detection of Mycobacterium in zebrafish Danio rerio. Dis Aquat Org 2013;104:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spence R, Gerlach G. Lawrence C, Smith C. The behavior and ecology of the zebrafish, Danio rerio. Biol Rev 2008:83:13–34 [DOI] [PubMed] [Google Scholar]
- 13.Castranova D, Lawton A, Lawrence C, Baumann DP, Best J, Coscolla J, et al. . The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 2011;8:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish 2007;4:21–40 [DOI] [PubMed] [Google Scholar]
- 15.Harper C, Lawernce C: The Laboratory Zebrafish. CRC Press, Boca Raton, FL and Smith, 2011 [Google Scholar]
- 16.Ramsay JM, Watral V, Schreck CB, Kent ML. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). J Fish Dis 2009;32:931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, et al. . Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Org 2011;95:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossteuscher S, Schmidt-Posthaus H, Schäfers C, Teigeler T, Segner H. Background pathology of the ovary in a laboratory population of zebrafish Danio rerio. Dis Aquat Org 2008;79:169–172 [DOI] [PubMed] [Google Scholar]
- 19.Ariëns Kappers CU. The meninges in Cyclostomes, Selachians, and Teleosts, compared with those in man. Proc R Acad Amst 1924;28:71–80 [Google Scholar]
- 20.Caruncho HJ, Da Silva PP. Alterations in the intermediate layer of goldfish meninges during adaptation to darkness. J Anat 1994:184:355–362 [PMC free article] [PubMed] [Google Scholar]