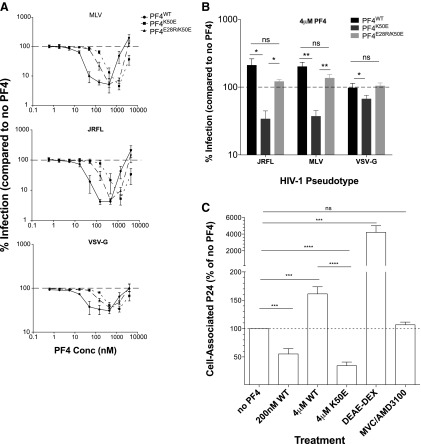
FIG. 5.
PF4 oligomeric state correlates with its biphasic activity on viral infection. (A) MAGI-R5 cells were infected with HIV-1 pseudoviruses bearing the glycoprotein of MLV (top), JRFL (middle), and VSV (bottom) in the absence or increasing presence of PF4WT, PF4K50E, or PF4E28R/K50E. (B) Virus entry was measured at high (4 μM) concentrations of PF4WT, PF4K50E, or PF4E28R/K50E. PF4WT enhanced infection of HIV-1JRFL and HIV-1MLV approximately twofold. PF4K50E significantly inhibited HIV-1JRFL, HIV-1MLV, and HIV-1VSV-G entry. PF4E28R/K50E restored the enhancement observed with PF4WT. PF4WT and PF4E28R/K50E did not significantly impact HIV-1VSV-G entry. (C) High concentrations of the monomer-favoring mutant PF4K50E significantly reduced viral binding to cells. All experiments were done in at least triplicate in each of at least three independent experiments. Error bars represent standard deviations. ns, p > 0.05; *p < 0.05; **p < 0.005; ***p < 0.0001.