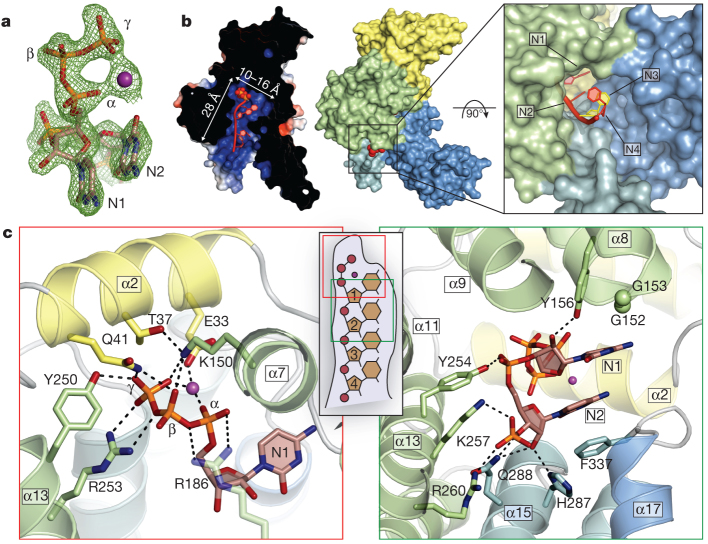
Figure 2. Structure of IFIT5 bound to PPP-RNA.
a, Fo– Fc electron-density map of the triphosphate and first two nucleotides contoured at 3.5σ before inclusion of RNA into the model. The metal ion is indicated with a purple sphere. b, Left, cross-section of the complex coloured by surface electrostatic potential. The triphosphate is shown as spheres and RNA nucleotides are shown in red. Middle, surface representation of IFIT5 bound to PPP-RNA coloured by subdomain. Protruding RNA is shown as red spheres. Right, close-up view looking down the axis of the RNA-binding pocket. c, Close-up view of the residues making specific contacts with the triphosphate group (left) and the first two nucleotides, N1 and N2 (right). Helices are coloured according to the subdomain to which they belong. Hydrogen bond and salt-bridge interactions are indicated with black dashed lines.