TO THE EDITOR: Acute myeloid leukemia (AML) with t(4;12) is rare, but its unique morphologic and clinical characteristics have been described in several reports [1,2,3,4,5,6,7,8]. Most cases show CD7 expression, low or absent myeloperoxidase activity, basophilia, unique blast morphology, dysplastic features, and poor prognosis [1,2,3,4,5,6,7,8]. Harada et al. reported that the incidence among adults is 0.6% [1,2]. ETV6, at 12p13, and CHIC2, at 4q12, have been reported to be involved in AML [5,8]. Here, we report 2 cases of AML with t(4;12) (q12;p13) which showed the common characteristics of AML with t(4;12).
Case 1
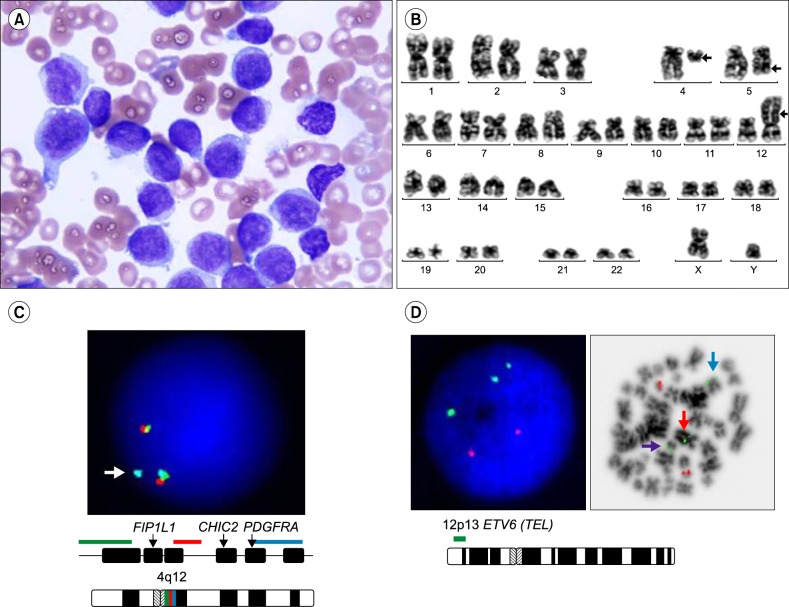
A 53-year-old male underwent a bone marrow (BM) examination following detection of circulating blasts. His initial complete blood count was hemoglobin 12.4 g/dL, white blood cell count 44.57×109/L (differential count: myeloblasts 99%, atypical lymphocytes 1%), and platelets 120×109/L. BM aspirates revealed 84% myeloblasts and dysmegakaryopoiesis, such as multinucleation and nuclear hypolobation. Myeloblasts were large and showed a fine and blocky chromatin pattern (Fig. 1A). Flow cytometric analysis revealed CD34, CD13, CD33, CD117, HLA-DR, and CD7 expression but was negative for myeloperoxidase. The karyotype was 46,XY,t(4;12)(q12;p13),del(5)(q22q35) in 20 metaphases (Fig. 1B). FISH analysis using a Vysis LSI 4q12 tricolor probe (Abbott Molecular, Des Plaines, IL, USA) showed 1 tri-color fusion, 1 orange/green fusion, and 1 aqua signal in 89% of cells. These results confirmed that the break point is at 4q12 and suggested that PDGFRA or CHIC2 may be involved (Fig. 1C). FISH analysis using a Vysis LSI ETV6/RUNX1 ES dual-color translocation probe (Abbott Molecular) showed 3 green signals in metaphase cells, suggesting an ETV6 gene rearrangement (Fig. 1D). The patient did not achieve complete remission after standard induction chemotherapy (idarubicin and cytarabin). Post-induction, 24% of all marrow nucleated cells were myeloblasts and the karyotype was 46,XY,t(4;12)(q12;p13), del(5)(q22q35)[2]/46,XY[2]. After reinduction using high-dose cytarabin and daunorubicin, blasts were not increased and a normal karyotype was detected (46,XY[17]). The patient underwent allogenic hematopoietic stem cell transplantation from an unrelated matched donor and has been in complete remission for 52 months.
Fig. 1. Case 1 (A) Bone marrow aspirates from patient 1. Large, round nucleus with a blocky chromatin pattern in agranular cytoplasm (Wright stain, ×1,000). (B) Karyotype of patient 1: 46,XY,t(4;12)(q12;p13),del(5)(q22q35)[20]. (C) FISH analysis using a Vysis LSI 4q12 tricolor probe showed cells with 1 tri-color fusion, 1 orange/green fusion, and 1 aqua signal (arrow). (D) FISH analysis using a Vysis LSI ETV6/RUNX1 ES probe showed 3 green and 2 orange signals in interphase and metaphase cells, revealing an ETV6 rearrangement [purple arrow: ETV6 signal on der(4)t(4;12), blue arrow: ETV6 signal on native chromosome 12, red arrow: ETV6 signal on der(12)t(4;12)].
Case 2
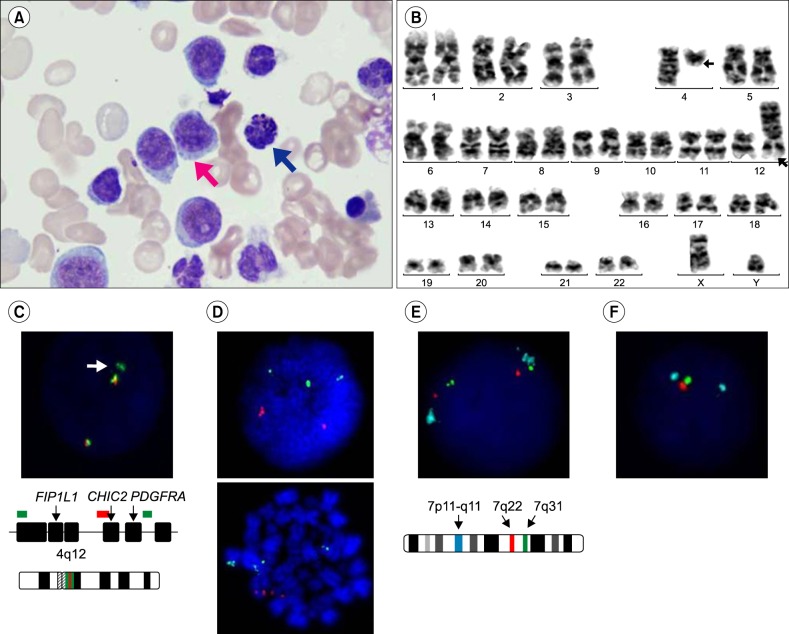
A 74-year-old male with a history of acute myocardial infarction presented at a local clinic complaining of melena. He was referred to the tertiary hospital following detection of myeloblasts on a peripheral blood smear. His initial complete blood count was hemoglobin 7.7 g/dL, white blood cell count 20.91×109/L (differential count: myeloblasts 45%, atypical lymphocytes 19%, and basophils 3%), and platelets 252×109/L. In BM aspirates, approximately 50% of all marrow nucleated cells were myeloblasts, which were large with a blocky chromatin pattern with prominent nucleoli (Fig. 2A). Basophils (3.8%) were also detected. Erythroid precursors showed minimal dysplastic features, such as multinuclearity, and myeloid precursors showed hypogranulation. Megakaryocytes were increased in number and showed dysplastic features, such as micromegakaryocytes and nuclear hypolobation. Flow cytometric analysis showed positivity for CD34, CD13, CD33, CD117, CD64, TdT, HLA-DR, aberrant CD7, and CD56 expression and was negative for myeloperoxidase. Cytogenetic analysis showed a karyotype of 46,XY,t(4;12)(q12;p13)[13] (Fig. 2B). FISH analysis using an XL FIP1L1/CHIC2/PDGFRA probe (Metasystems, Altlussheim, Germany) showed 2 orange/green fusions and 1 green signal in 82% of cells, indicating a translocation involving a breakpoint at 4q12 (Fig. 2C). An LSI ETV6/RUNX1 ES dual-color translocation probe (Abbott Molecular) showed 3 green signals, suggesting an ETV6 gene rearrangement in metaphase cells (Fig. 2D).
Fig. 2. Case 2 (A) Bone marrow aspirates from patient 2. Large, round nucleus with a blocky chromatin pattern with a prominent nucleolus (red arrow). Basophil (blue arrow) (Wright stain, ×1,000). (B) Karyotype of patient 2: 46,XY,t(4;12)(q12;p13)[13]. (C) FISH analysis using an XL FIP1L1/CHIC2/PDGFRA probe showed 2 orange/green fusions and 1 green signal (arrow). (D) FISH analysis using a Vysis LSI ETV6/RUNX1 ES probe showed 3 green and 2 orange signals in interphase and metaphase cells, revealing an ETV6 rearrangement. (E) FISH analysis using an XL del(7)(q22q31) tri-color probe showed 2 aqua, 2 green, and 2 orange signals at the initial diagnosis. (F) FISH analysis using an XL del(7)(q22q31) tri-color probe showed 2 aqua, 1 green, and 1 orange signal at the follow-up, showing deletion signals for 7q22-7q31.
The patient received 3 courses of decitabine single therapy, based on his age and comorbidities. Following the therapy, blasts were still detected and the karyotype was 46,XY,t(4;12)(q12;p13)[37]/46,sl,del(7)(q22)[3]. A subclone harboring a 7q deletion was found to have evolved. Blasts and trilineage dysplasia remained after 14 courses of decitabine treatment. The patient then received induction chemotherapy with idarubicin and cytarabine, though BM aspirates still showed 50% blasts and dysplastic features. FISH analysis using an XL del(7)(q22q31) tri-color probe (Metasystems) was performed with the initial and follow-up BM to confirm the clonal evolution of del(7q). The initial sample showed no deletion signals (Fig. 2E); however, the follow-up sample showed deletion signals for 7q22-7q31 (Fig. 2F).
DISCUSSION
t(4;12)(q11-q13;p12-p13) was previously described as a recurrent translocation in different types of acute leukemia. Eighteen cases of AML have been reported, along with 3 cases of acute lymphoblastic leukemia in children [9]. The cases that presented as acute myeloid leukemia showed unique blasts, with "pseudo-lymphoid" or "mature lymphoid" morphology, and with a blocky chromatin pattern resembling that of lymphocytes. Dysplastic features are also frequently noted. The 2 cases presented here both showed the morphology described above.
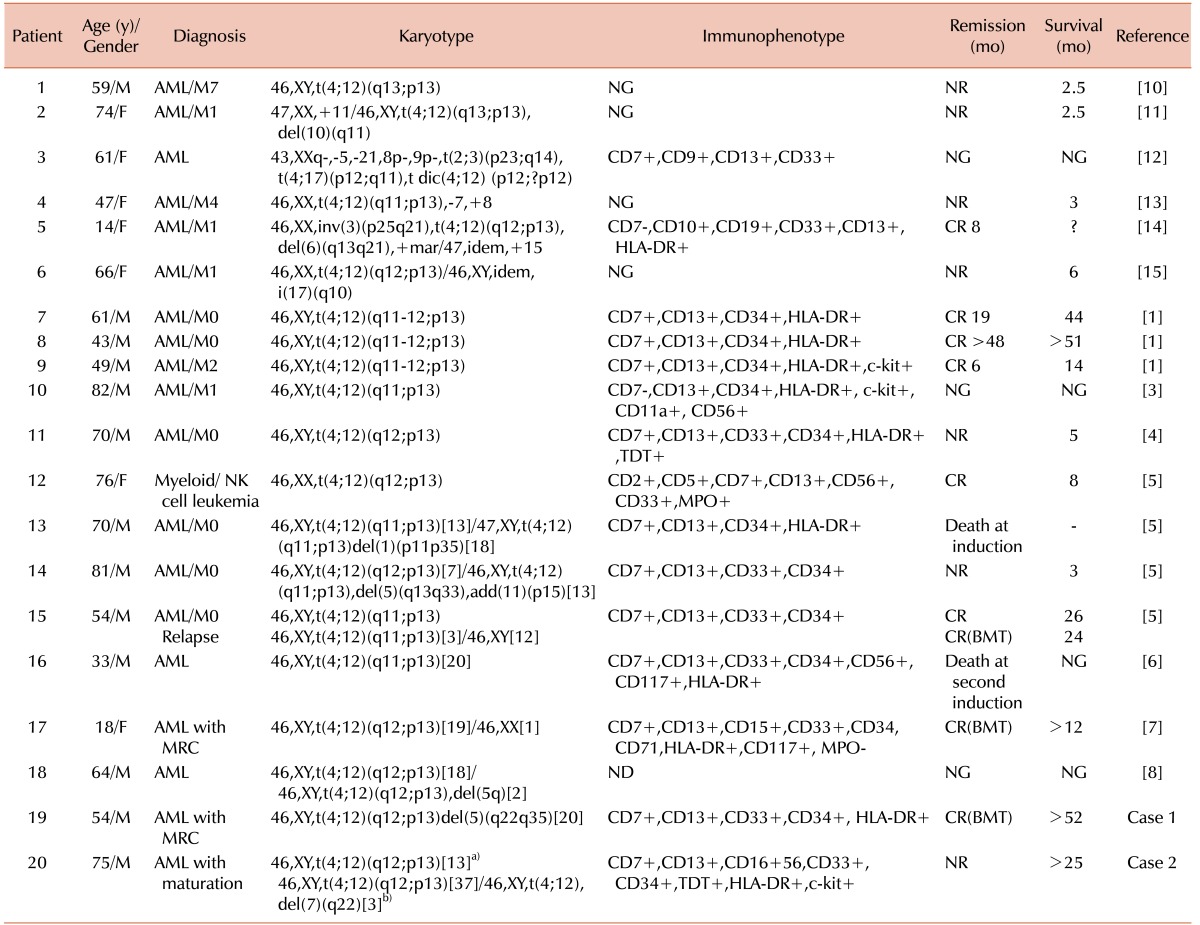
The translocation presented as either a single chromosomal abnormality or combined with other abnormalities (Table 1) [1,3,4,5,6,7,8,10,11,12,13,14,15]. The karyotype of the first patient was 46,XY, t(4;12)(q12;p13),del(5)(q22q35), and del(5q) is a common chromosomal abnormality in myelodysplastic syndrome and in AML with myelodysplasia-related changes. Basophilia was not observed in this case. Other common characteristics, such as CD7 expression, negative myeloperoxidase activity, and failure of the first induction chemotherapy were observed in the first case. As in previous reports, intensive treatment or hematopoietic stem cell transplantation were curative [5,7]. In the second case, t(4;12) presented as a single abnormality, which showed development of a subclone with a 7q deletion as the disease progressed. Several previously described features, such as CD7 expression, absence of myeloperoxidase activity, and basophilia were present. Despite administration of decitabine therapy, the usual treatment for myelodysplastic syndromes and AML for elderly patients, and additional induction chemotherapy, this patient has not entered remission.
Table 1. Patients with t(4;12) in acute myeloid leukemia.
a)The karyotype of patient 2 at diagnosis; b)The karyotype of patient 2 after 3 courses of decitabine therapy.
Abbreviations: AML, acute myeloid leukemia; AUL, acute unclassified leukemia; Tx, treatment; NK, Natural killer; MRC, myelodysplasia-related changes; NG, not given; ND, not done; NR, no remission; CR, complete remission; BMT, bone marrow transplantation.
ETV6 (also known as TEL) is involved in several malignancies, including pre-B cell ALL with t(12;21)(p13;q22) and CMML with t(5;12)(q33;p13) [8]. A previous study mapped the breakpoints of patients with t(4;12) as ETV6 at 12p13 and CHIC2 at 4q12, resulting in the formation of CHIC2-ETV6 fusion transcripts [5] and the involvement of ETV6 [8]. The CHIC2-ETV6 fusion transcript has been suggested to be involved in the leukemogenic process [5]. FISH analysis of these cases also suggested rearrangements of ETV6 and CHIC2.
In summary, the 2 cases discussed here shared most of the common characteristics of AML with t(4;12)(q11-q13;p12-p13), as well as some different features. When myeloblasts show a distinctive blocky chromatin pattern or "mature lymphoid" morphology, t(4;12) should be suspected and the appropriate evaluation should follow. Intensive chemotherapy or early hematopoietic stem cell transplantation should be considered. Further studies of this rare but recurrent cytogenetic abnormality will guide proper diagnosis and management.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Harada H, Asou H, Kyo T, et al. A specific chromosome abnormality of t(4;12)(q11-12;p13) in CD7+ acute leukaemia. Br J Haematol. 1995;90:850–854. doi: 10.1111/j.1365-2141.1995.tb05206.x. [DOI] [PubMed] [Google Scholar]
- 2.Harada H, Harada Y, Eguchi M, Dohy H, Kamada N. Characterization of acute leukemia with t(4;12) Leuk Lymphoma. 1997;25:47–53. doi: 10.3109/10428199709042495. [DOI] [PubMed] [Google Scholar]
- 3.Sainty D, Arnoulet C, Mozziconacci MJ, De Pina JJ, Garnotel E, Lafage-Pochitaloff M. t(4;12)(q11;p13) in a CD7-negative acute myeloid leukaemia. Br J Haematol. 1997;96:210–212. doi: 10.1046/j.1365-2141.1997.d01-3153.x. [DOI] [PubMed] [Google Scholar]
- 4.Ma SK, Lie AK, Au WY, Wan TS, Chan LC. CD7+ acute myeloid leukaemia with 'mature lymphoid' blast morphology, marrow basophilia and t(4;12)(q12;p13) Br J Haematol. 1997;99:978–980. [PubMed] [Google Scholar]
- 5.Cools J, Bilhou-Nabera C, Wlodarska I, et al. Fusion of a novel gene, BTL, to ETV6 in acute myeloid leukemias with a t(4;12)(q11-q12;p13) Blood. 1999;94:1820–1824. [PubMed] [Google Scholar]
- 6.Chauffaille Mde L, Fermino FA, Pelloso LA, Silva MR, Bordin JO, Yamamoto M. t(4;12)(q11;p13): a rare chromosomal translocation in acute myeloid leukemia. Leuk Res. 2003;27:363–366. doi: 10.1016/s0145-2126(02)00172-8. [DOI] [PubMed] [Google Scholar]
- 7.Manabe M, Nakamura K, Inaba A, et al. A rare t(4;12)(q12;p13) in an adolescent patient with acute myeloid leukemia. Cancer Genet Cytogenet. 2010;200:70–72. doi: 10.1016/j.cancergencyto.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Al-Kali A, Cherry M, Kimmell K, et al. A case of acute myeloid leukemia initially treated as chronic lymphocytic leukemia: what do we know about t(4;12)(q12;p13)? Cancer Genet Cytogenet. 2010;203:348–351. doi: 10.1016/j.cancergencyto.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 9.van der Plas DC, Dekker I, Hagemeijer A, Hooijkaas H, Hählen K. 12p chromosomal aberrations in precursor B childhood acute lymphoblastic leukemia predict an increased risk of relapse in the central nervous system and are associated with typical blast cell morphology. Leukemia. 1994;8:2041–2046. [PubMed] [Google Scholar]
- 10.Ohyashiki K. Nonrandom cytogenetic changes in human acute leukemia and their clinical implications. Cancer Genet Cytogenet. 1984;11:453–471. doi: 10.1016/0165-4608(84)90026-8. [DOI] [PubMed] [Google Scholar]
- 11.Berger R, Flandrin G, Bernheim A, et al. Cytogenetic studies on 519 consecutive de novo acute nonlymphocytic leukemias. Cancer Genet Cytogenet. 1987;29:9–21. doi: 10.1016/0165-4608(87)90026-4. [DOI] [PubMed] [Google Scholar]
- 12.Matutes E, Foroni L, Amin S, et al. 'Pseudo-lymphoid' leukaemia with unusual features: ultrastructural, immunological, cytogenetic and molecular studies. Eur J Haematol. 1987;38:303–309. doi: 10.1111/j.1600-0609.1987.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 13.den Nijs van Weert JI, Beverstock GC, Kievits T, Haak HL, Havik-Bogaard FC, Leeksma CH. der(1)t(1;9): a specific chromosome abnormality in polycythemia vera? Cytogenetic and in situ hybridization studies. Cancer Genet Cytogenet. 1989;40:121–127. doi: 10.1016/0165-4608(89)90153-2. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Raimondi SC, Head DR, et al. Characterization of childhood acute leukemia with multiple myeloid and lymphoid markers at diagnosis and at relapse. Blood. 1991;78:1327–1337. [PubMed] [Google Scholar]
- 15.Translocations involving 12p in acute myeloid leukemia: association with prior myelodysplasia and exposure to mutagenic agents. United Kingdom Cancer Cytogenetics Group (UKCCG) Genes Chromosomes Cancer. 1992;5:252–254. doi: 10.1002/gcc.2870050313. [DOI] [PubMed] [Google Scholar]