Abstract
Increasing evidence suggests that botulinum neurotoxins (BoNTs) delivered into the skin and muscle in certain human and animal pain states may exert antinociceptive efficacy though their uptake and transport to central afferent terminals. Cleavage of soluble N-methylaleimide-sensitive attachment protein receptor (SNAREs) by BoNTs can impede vesicular mediated neurotransmitter release as well as transport/insertion of channel/receptor subunits into plasma membranes, an effect that can reduce activity-evoked facilitation. Here, we explored the effects of intraplantar Botulinum toxin- B (BoNT-B) on peripheral inflammation and spinal nociceptive processing in an inflammatory model of pain. C57BL/6 mice (male) received unilateral intraplantar BoNT (1U, 30 μL) or saline prior to intraplantar carrageenan (20 μL, 2%) or intrathecal N-methyl-D-aspartate (NMDA), substance P or saline (5 μL). Intraplantar carrageenan resulted in edema and mechanical allodynia in the injected paw and increased phosphorylation of a glutamate subunit (pGluA1ser845) and a serine/threonine-specific protein kinase (pAktser473) in spinal dorsal horn along with an increased incidence of spinal c-Fos positive cells. Pre-treatment with intraplantar BoNT-B reduced carrageenan evoked: i) allodynia, but not edema; ii) pGluA1 and pAkt and iii) c-Fos expression. Further, intrathecal NMDA and substance P each increased dorsal horn levels of pGluA1 and pAkt. Intraplantar BoNT-B inhibited NMDA, but not substance P evoked phosphorylation of GluA1 and Akt. These results suggest that intraplantar toxin is transported centrally to block spinal activation and prevent phosphorylation of a glutamate receptor subunit and a kinase, which otherwise contribute to facilitated states.
Keywords: Botulinum toxin, spinal sensory processing, carrageenan, SNARE, VAMP, spinal cord, dorsal root ganglion
Graphical abstract

1. Introduction
Botulinum toxin (BoNT) serotypes are constructed of heavy (HC) and light chain (LC) components. After HC dependent uptake, the cleaved LC acts to cleave consensus sequences present on SNARE (soluble N-methylaleimide-sensitive attachment protein receptor) synaptic proteins (Montecucco & Molgo, 2005). The BoNT-B serotype targets VAMP (vesicle-associated membrane protein), which is found on neurotransmitter vesicles and may also be located on endosomes containing membrane receptors (Humeau et al., 2000; Montecucco & Molgo, 2005; Ramachandran & Yaksh, 2014; Schenk et al., 2003). Given the diverse role of SNAREs in cytosolic trafficking, BoNTs can block endosomal fusion and synaptic release of neurotransmitters and may also inhibit activity-induced insertion or deletion of receptor and channel proteins being trafficked to the membrane (Kakegawa & Yuzaki, 2005; Marino et al., 2014; Pellett et al., 2015; Shimizu et al., 2012). Importantly, BoNT is taken up by peripheral afferent terminals where it cleaves local SNAREs and undergoes fast spinopetal axon transport from the peripheral terminal to further cleave SNAREs within dorsal root ganglia and in the central primary afferent terminals to block both central and peripheral neurotransmitter release (Antonucci et al., 2008; Lawrence et al., 2012; Marino et al., 2014; Restani et al., 2011). It has been increasingly appreciated that this peripheral uptake and central transport may lead to prominent changes in the processing of spinal nociceptive signals (Bach-Rojecky & Lackovic, 2009; Filipovic et al., 2012; Marino et al., 2014; Matak et al., 2011; Pellett et al., 2015).
The decrease in spinal afferent neurotransmitter release is most frequently postulated for the anti-hyperalgesic effects observed following peripheral BoNT injection. Indeed there is a substantial literature describing these effects in both pre-clinical and human studies (Favre-Guilmard et al., 2009; Huang et al., 2011; Tugnoli et al., 2007). Puzzlingly, these anti-hyperalgesic effects are typically observed in the absence of changes in proprioceptive or low threshold inputs and a maintenance of responses to brief high intensity stimuli, e.g. the preservation of acute thermal withdrawal thresholds in rodents (Marino et al., 2014). Thus, the mechanism of action of BoNTs must include aspects that are unique to sensitization and not consist simply of a non-selective reduction of afferent neurotransmitter release. Intraplantar (i.pl.) BoNT is also reported to decrease local flare and plasma extravasation in response to local capsaicin (Marino et al., 2014), however little is known about other potential anti-inflammatory effects. In addition to presynaptic effects limited to the primary afferent fiber, BoNT has been postulated to travel trans-synaptically from the central terminals of the injected afferents where it may be either endocytosed into postsynaptic neurons or into primary afferent fibers with terminals adjacent to the injected fibers (Filipovic et al., 2012; Kim et al., 2015; Marino et al., 2014; Ramachandran et al., 2015). The present experiments were designed to address new aspects of BoNT-B local effects on peripheral inflammation as well as to examine effects upon central events that result from persistent small afferent input, as occurs after peripheral inflammation.
2. Materials and Methods
Adult male C57BL/6 mice (20-25g, Harlan Industries, Indianapolis IN) were housed with food and water provided ad libitum, under a 12 h light/dark cycle with up to 4 mice per cage. Animals were kept for a minimum of 2 days before use. Experiments were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Use Committee of the University of California, San Diego approved all protocols. All procedures were conducted between 9:00 and 16:00 h.
2.1. Intraplantar Botulinum Toxin-B Injection
Anesthesia was induced with 2.5 % isoflurane delivered in equal parts O2 and room air in a closed chamber until a loss of the righting reflex was observed. A Hamilton syringe attached to a calibrated length of polyethylene tubing and a 30-gauge needle were used to administer BoNT-B (Rimabotulinumtoxin B, Myobloc ®, Solstice Neurosciences, Louisville, KY) or sterile saline subcutaneously to the mid-plantar hindpaw in a 30 μL volume. The dose of BoNT-B in the present study was chosen based on our recent work demonstrating the dose dependent effects of intraplantar BoNT-B on formalin flinching. In these studies, we found that 1 unit/30μL given intraplantar produced significant cleavage of DRG SNAREs and inhibition of Phase 2 flinching without morbidity (Marino et al., 2014). BoNT-B was freshly prepared from stock solutions of 5000 U/mL and diluted in 0.9% saline to the final dose of 1 unit/30 μL. Solutions were stored at 4°C and warmed to room temperature before use. Unless otherwise noted, intraplantar BoNT-B treatment was always given two days prior to any treatment mentioned below.
2.2. Intraplantar Carrageenan Injection
Two days after BoNT-B or vehicle pre-treatment, anesthesia was induced as above. Lambda carrageenan (Wako Pure Chemical Industries, Ltd) (2%, 20 μL) was subcutaneously injected into the plantar surface of one or both hind paws. In instances of unilateral carrageenan, an equal amount of isotonic saline was injected into the contralateral paw.
2.3. Intrathecal N-methyl-D-aspartate (NMDA) or substance P (sP) Injection
To permit direct activation of spinal nociceptive circuitry, animals received intrathecal delivery of the glutamate receptor agonist N-methyl-D-aspartate (NMDA) (M3262, Sigma Aldrich, St. Louis, MO) or the NK1 receptor agonist substance P (sP) (S6883, Sigma Aldrich, St. Louis, MO). Two days after BoNT-B or vehicle pre-treatment, anesthesia was induced and maintained with 2.5% isoflurane. Maintenance anesthesia was administered via a nosecone. Mice were then intrathecally (i.t.) injected between the L5 and L6 vertebrae with 5 μL of NMDA (0.25nM), sP (3.71 pM) or saline. A flick of the tail upon insertion of the needle was taken to indicate appropriate needle placement. Mice remained anesthetized and the spinal cords were harvested 5 min post-injection for western blot analysis of pAkt and pGluA1.
2.4. Tactile Allodynia
Mechanical withdrawal thresholds were measured on both hindpaws using a set of von Frey filaments with bending forces ranging from 0.04-2.00 g. Filaments were applied to the mid-plantar hindpaw near the injection sites. The up-down method (Chaplan et al., 1994) was used to determine thresholds just prior to a BoNT-B injection on the left hindpaw, before unilateral i.pl. carrageenan, administered to the left hindpaw, and at 60, 90, 120, 180 and 240 min post-carrageenan. Prior to testing sessions, mice were acclimated to the test chambers for 1h. Paw thickness was measured with a micrometer before, and 4 h after carrageenan/saline injections as an index of edema/inflammation.
2.5. Immunohistochemistry c-Fos
Four hours post-carrageenan, mice were deeply anesthetized with 2.5% isoflurane and transcardially perfused with 0.9% saline followed by ice cold 4% paraformaldehyde (PFA). The L4/5 spinal cords were harvested, post fixed overnight with PFA and then after cryoprotection in 30% sucrose, 30 μm sections were collected. Immunocytochemistry for c-Fos was performed on free-floating sections using standard avidin/biotin peroxidase methods. Tissue was labeled using rabbit anti c-Fos (1:20,000, # PC38, Calbiochem, La Jolla, CA) incubated overnight at room temperature, rinsed with phosphate buffered saline (PBS) and then incubated with a goat anti-rabbit biotinylated secondary antibody. An observer blinded to the treatment counted c-Fos positive cells in L4/L5 (laminae I-II and III-V) using light microscopy.
2.6. Western Blots
2.6.1. Tissue harvest
For VAMP analysis, mice received unilateral i.pl. injections of BoNT-B or contralateral i.pl. injections of saline. 2 days after injection, the animals were deeply anesthetized and decapitated and tissues (spinal cord and DRG) were harvested as described previously (Marino et al., 2014). For pAkt and pGluA1 analysis, mice that were pre-treated with unilateral i.pl. BoNT-B or saline (2 days prior) received either bilateral i.pl. carrageenan or i.t. NMDA or i.t. sP. In i.pl. carrageenan treated group tissues were harvested at 1 hr post-injection, whereas in i.t. NMDA or sP treated group, tissues were collected after 5 min post-injection. Briefly, spinal cords were collected via hydroextrusion (injection of 2 mL of iced saline though a short blunt 20 gauge needle placed into the spinal canal following decapitation). The lumbar enlargement was dissected and then separated into dorsal or ventral halves, or into 4 quadrants. Dorsal spinal cord sections were placed in vials containing 75 μL cold extraction buffer with added protease inhibitor cocktail (P8340, 1:100) and phosphatase inhibitor cocktail 2 and 3 (1:100, P5726 and P2850). All agents were from Sigma-Aldrich, St. Louis, Mo. USA. In some experiments, after spinal cord tissue harvest, the L5-L6 DRGs on each side were combined and frozen as for the spinal cord. Vials were flash frozen on dry ice and kept at -70°C until analysis.
2.6.2. VAMP, pAkt and pGluA1 staining
Tissues were defrosted and homogenized by sonication followed by centrifugation at 14,500 rpm for 15 min at 4°C. Supernatant was then collected and processed by SDS-PAGE and transferred to nitrocellulose membranes (iBlot, IB301001, Invitrogen). Membranes (DRG and spinal cord samples) were incubated with rabbit polyclonal anti-VAMP 1,2,3 (104203, 1:1000, Synaptic Systems, Gottingen, Germany) and anti-rabbit Horseradish Peroxidase (Cell Signaling Technology, Beverly, MA, USA). Membranes were then stripped and re-probed with β-actin (1:50,000; Sigma-Aldrich), which was used as a loading control. This VAMP antibody detects the intact protein of all three VAMP isoforms. Only VAMP 1 and 2 have been reported in neurons with VAMP 1 predominating in the spinal cord (Elferink et al., 1989) and VAMP 2 being found exclusively in the DRG (Marino et al., 2014). In the mouse, VAMP 1 and 2 are both cleaved by BoNT-B (Humeau et al., 2000; Schiavo et al., 1992), thus, decreases in either protein implies BoNT-B activity. Other primary antibodies used were pAkt ser473 (#9271, rabbit, 1:1000; Cell Signaling) and pGluA1 ser845 (04-1073, rabbit, 1:000; Millipore, Temecula, CA). Membranes were probed first for the pGluA1, then pAkt and finally β-actin. The two phosphorylated compounds were employed as indices of dorsal horn neuronal sensitization. All blots shown for an individual protein were run on the same gel and subjected to identical developing conditions.
2.7. Statistics
Data are presented as means ± SEM. All tests were performed with Prism 6 for OS-X (GraphPad Software, La Jolla, CA, USA). A 2-way ANOVA for repeated measures with post-hoc Bonferroni's multiple comparisons test was used to analyze the behavioral data Fig 1A. Paw thickness data in Fig 1B and c-Fos data in Fig 2 were analyzed with paired t-tests; un-paired t-tests were used for the Western blot data. p≤ 0.05 (2 tailed) was considered to be significant in all cases
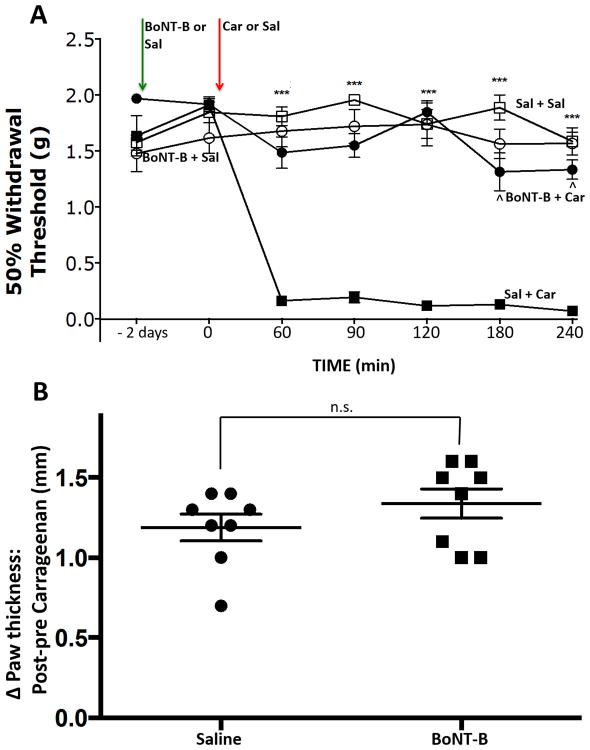
Figure 1. Effect of intraplantar BoNT-B on carrageenan induced mechanical hypersensitivity and edema.
A) Intraplantar pre-treatment with BoNT-B reduced the magnitude and delayed the onset of mechanical hypersensitivity induced by i.pl. carrageenan. *** p≤ 0.001 BoNT-B + carrageenan vs saline + carrageenan. ˆ p≤0.001 BoNT-B + carrageenan vs baseline. Two-way ANOVA for repeated measures with post-hoc Bonferroni's multiple comparisons test. B) In contrast, increases in paw thickness following carrageenan were unaffected by the BoNT-B pre-treatment. Paired t-tests. N=8/gp. n.s: non significant, BoNT-B: botulinum toxin B.
Figure 2. Effect of intraplantar BoNT-B on c-Fos expression evoked by ipsilateral intraplantar carrageenan in the spinal cord dorsal horn.
A) Representative images of contralateral (contra) and ipsilateral (ipsi) dorsal horn labelled with c-Fos positive cells following saline or BoNT-B pre-treatment in the ipsilateral carrageenan treated group. B) Plot represents the total number of c-fos positive neurons in the contra and ipsi dorsal horn at 2 hrs after unilateral carrageenan and following the pre-treatment with ipsilateral saline or BoNT-B in superficial (lamina I &II) and deep (lamina III & IV) laminae. Unilateral paw carrageenan increased c-Fos expression in the ipsilateral dorsal horn as compared to the contralateral saline treated side. This was true for both superficial and deep laminae p≤ 0.004 and p≤ 0.02, respectively. However, following BoNT-B pre-treatment, significance was lost for both superficial and deep dorsal horn, p≤0.375 and 0.327 respectively. Paired t-tests. N=3-4/gp. BoNT-B: botulinum toxin B.
3. Results
3.1. Mechanical allodynia and edema
Following unilateral i.pl. carrageenan injections, i.pl. saline pre-treated paws displayed a marked allodynia with thresholds falling from pre-carrageenan thresholds of 1.91g ± 0.06 to 0.16g ± 0.4, by 60 min post-carrageenan, (Fig 1A: p≤0.001, F=21.71, Df=6, 2-way ANOVA for repeated measures). Mechanical withdrawal thresholds remained depressed, indicating hypersensitivity for the remaining 3 h of the experiment. Intraplantar BoNT-B had no effect on baseline withdrawal thresholds as compared to i.pl. saline measured 2 days post-injection. However, with BoNT-B pre-treatment, there was a significant reduction in carrageenan-induced mechanical hypersensitivity, e.g. tactile thresholds now only decreased from 1.92g ± 0.4 to 1.49g ± 0.1 (p≤0.0001, F=112.1, Df=3, 2-way ANOVA for repeated measures). At all post-carrageenan time points, BoNT-B pre-treated paws had mechanical thresholds significantly above those of the saline pre-treated paws (p≤0.001; Bonferonni's post-hoc test). Irrespective of their pre-treatment, the contralateral (untreated) paw displayed no changes in mechanical withdrawal threshold from control (p=0.88).
Pre-injection paw thickness was similar for both saline and BoNT-B pre-treated groups, (1.64 mm ± 0.03 and 1.66 mm ± 0.03, respectively) and carrageenan delivered after either pre-treatment resulted in significant swelling (p≤ 0.0001, dF=7). Thus, despite marked differences in pain behavior, paw thickness at 4 h post-carrageenan was similar in both the saline and BoNT-B pre-treated animals (Fig 1B). The change in paw thickness over the course of the experiment was 1.19 mm ± 0.08 for the saline pre-treated paws and 1.34 mm ± 0.09 for the BoNT-B pre-treated paws (p=895, F=1.108, dF= 7). These data indicate that peripheral BoNT-B injection blocks inflammation-induced behavioral hypersensitivity without altering the magnitude of the carrageenan-evoked peripheral inflammatory response.
3.2. c-Fos
Cells expressing carrageenan-induced c-Fos in the dorsal horn were counted as an objective measure of spinal sensitization and nociception. Positive cells were counted separately in the superficial (laminae I and II) and deep (laminae III-V) dorsal horns. In saline pre-treated animals (n=4), the superficial dorsal horn ipsilateral to the carrageenan injection expressed on average 364 % more c-Fos positive neurons compared to the contralateral side. Absolute counts were 11.42 ± 1.08 and 3.13± 0.07, respectively (p≤ 0.004, paired t-test). In BoNT-B pretreated animals (n=3) this percentage was reduced to 135.6%; absolute counts were 7.31 ± 1.37 and 5.72± 1.44 (p≥ 0.05, paired t-test). This indicates that peripheral injection of BoNT-B reduced the central effects of the carrageenan-induced barrage of activity necessary for spinal c-Fos expression. Fos positive cell counts in the deep dorsal horn mirrored findings of the superficial laminae as carrageenan induced a 182% increase on the ipsilateral side in saline pre-treated animals, with absolute counts 17.54± 2.21 on the carrageenan side and 9.99 ± 1.5 on the contralateral side (paired t-test; p≤ 0.02). This increase was lost in the BoNT-B pre-treated animals, which displayed only a 117% increase on the carrageenan side. Absolute counts were 8.28± 2.02 and 6.86± 0.97, respectively (paired t-test; p ≥ 0.05).
3.3. Phosphorylation of dorsal horn Akt and GluA1
We used phosphorylation of dorsal horn Akt and the AMPA subunit GluA1 as measures of spinal sensitization [6,4,2]. We chose to quantify pAkt ser473 and pGluA1 ser845, as our previous experience has shown that both are phosphorylated after i.pl. carrageenan (Choi et al., 2010). Bilateral paw carrageenan induced a 298% increase in pAkt ser473 compared to saline-injected control animals (cont) (n=4; p=0.0036; unpaired t-test, Fig 3A) - this effect is comparable to our previous results in the rat (Choi et al., 2010). In the same animals, carrageenan induced a 302% increase in pGluA1 ser845 compared to control animals (p=0.0074). Despite the similar increases there were no significant correlations between levels of pAkt and pGluA1 in individual animals (data not shown).
Figure 3. Effect of intraplantar BoNT-B pre-treatment on intraplantar carrageenan induced increase in pAkt and pGluA1 expression.
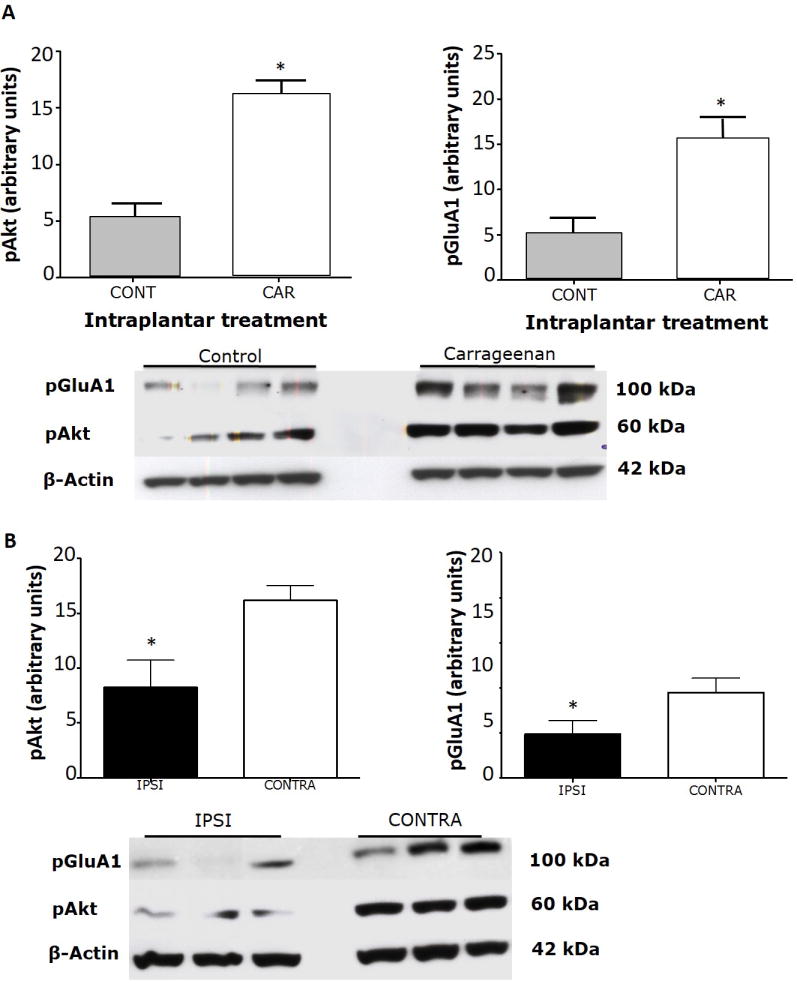
A) Bilateral intraplantar carrageenan (car) induced a significant increase in pAkt and pGluA1 compared to control (cont) in the dorsal half of the lumbar spinal cord. Tissue was sampled 1 h after the carrageenan injection. Histogram depicts the relative expression of pAkt and pGluA1 expression quantified by densitometric measurement. Representative Western blots are shown below the graphs. B) When the experiment was repeated with an i.pl. pre-treatment of BoNT-B in one paw (ipsi) and saline in the other, 2 days prior to the carrageenan injection, the levels of pAkt and pGluA1 in the ipsilateral dorsal quadrant of the spinal cord were significantly suppressed with respect to the saline pre-treated animals. Histogram depicts the relative expression of pAkt and pGluA1 expression quantified by densitometric measurement. Representative Western blots are below each set of graphs. * p≤0.01. Unpaired t-tests. N=4/gp. BoNT-B: botulinum toxin B, pAkt: phosphorylated Akt, pGluA1: phosphorylated GluA1.
When bilateral carrageenan injections followed a unilateral BoNT-B pre-treatment, expression of both pAkt and pGluA1 was consistently lower on the dorsal horn side ipsilateral to the BoNT-B injection (Fig 3B). Means of phosphorylation levels were 33% and 11% lower respectively of the side injected with i.pl. saline (n=9; p=0.027 for pAkt and p=0.016 for pGluA1; unpaired t-tests).
While these data demonstrate that i.pl. BoNT-B blocks central sensitization, the decrease in phosphorylation GluA1 and Akt, as well as the c-Fos data, could be explained by a loss of primary afferent drive following carrageenan administration, presumably due to the BoNT blockade of neurotransmitter release from the central terminals of the primary afferent fibers.
Conceptually the next step was to determine whether peripherally administered BoNT-B could block activation and subsequent sensitization of dorsal horn neurons that was independent of neurotransmitter release from the primary afferent fiber. We therefore used intrathecal administration of NMDA to elicit phosphorylation of Akt and GluA1 to address this issue. We designed this as a negative control, as we believed that if BoNT-B reached the post-synaptic neuron, it might block activity-induced insertion of the GluA1-containing endosomes into the plasma membrane, but that it would not block the earlier phosphorylation within the cytoplasm. For this experiment we used 3 groups; (1) i.pl. saline followed in 2 days by i.t. saline, (2) i.pl. saline followed by i.t. NMDA and (3) i.pl. BoNT-B followed by i.t. NMDA (n=4-5 per group). As expected, i.t. NMDA elicited clear increases in both pAkt (p≤0.022) and pGluA1 (p≤0.012) in the dorsal spinal cord (Fig 4). What was not anticipated was that ipsilateral BoNT-B pre-treatment blocked the NMDA-evoked phosphorylation response (p≤0.05 for both). Post hoc testing indicated significant differences between the i.pl. saline pre-treated/i.t. NMDA tissue and both the i.pl saline/i.t saline and i.pl. BoNT-B/i.t. NMDA groups (Bonferroni's multiple comparisons test). We then replicated this experiment, and achieved similar results, Fig 4 illustrates the combined results of both series (pAkt: p≤0.011, F=5.568, n=8-9; pGluA1: p≤0.004, F=7.158, n=8-9).
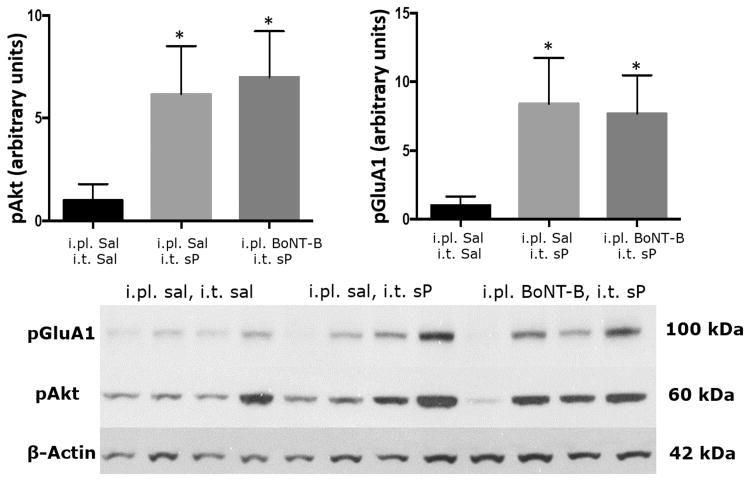
Figure 4. Effect of intraplantar BoNT-B pre-treatment on intrathecal NMDA induced increase in pAkt and pGluA1 expression.
Intrathecal NMDA elicited a significant increase in both pAkt and pGluA1 in the dorsal spinal cord within the lumbar enlargement compared to intrathecal saline. Tissue was sampled 5 min after i.t. injection. However, pre-treatment with i.pl. BoNT-B, reduced the dorsal spinal cord levels of pAkt and pGluA1 and were no longer different from those seen after i.t. saline. Histogram depicts the relative expression of pAkt and pGluA1 expression quantified by densitometric measurement. Representative Western blots are below each set of graphs. *p≤0.01 as compared to i.pl. saline/i.t. saline, **p≤0.05 as compared to i.pl. saline/i.t. NMDA, ***p≤0.001 i.pl. saline/i.t. saline. Unpaired t-tests. N=8-9/gp. BoNT-B: botulinum toxin B, pAkt: phosphorylated Akt, pGluA1: phosphorylated GluA1, NMDA: N-methyl-D-aspartate
As this was an unexpected result, we repeated these experiments substituting i.t. substance P for the NMDA delivery. Intrathecal administration of sP elicited an increase in dorsal spinal cord expression of both pAkt and pGluA1 (Fig 5; p=0.043, and p=0.049, respectively). In contrast to the previous NMDA results, i.pl. BoNT-B administered 2 days prior to the sP had no effect on the outcome and resulted in no change in sP-induced levels of the phosphorylated form of either the kinase (p=0.868) or the AMPA receptor subunit (p=0.726) within the spinal cord, both were still significantly elevated compared to tissue taken from saline injected animals (p=0.04 and p=0.018, respectively).
Figure 5. Effect of intraplantar BoNT-B pre-treatment on intrathecal substance P (sP) induced increase in pAkt and pGluA1 expression.
Intrathecal sP elicited a significant increase in both pAkt and pGluA1 in the dorsal spinal cord within the lumbar enlargement compared to intrathecal saline. Tissue was sampled 5 min after i.t. injection. Pre-treatment with i.pl. BoNT-B did not affect the dorsal spinal cord levels of pAkt and pGluA1 on the response to sP. Histogram depicts the relative expression of pAkt and pGluA1 expression quantified by densitometric measurement. Representative Western blots are below each set of graphs. *P≤0.05 as compared to i.pl. saline/i.t. saline. Unpaired t-test. N=7-8. BoNT-B: botulinum toxin B, pAkt: phosphorylated Akt, pGluA1: phosphorylated GluA1, sP: substance P
3.4. Expression of VAMP
When pooled DRGs from animals that received unilateral i.pl. BoNT-B injections were compared to contralateral i.pl. saline injections there was a small, but significant decrease in VAMP2 on the BoNT-B injected side, (p=0.03; n=5/gp, Fig 6A). We interpret these data to mean that BoNT-B, in an active form, reaches the ipsilateral DRGs to cleave SNARE proteins.
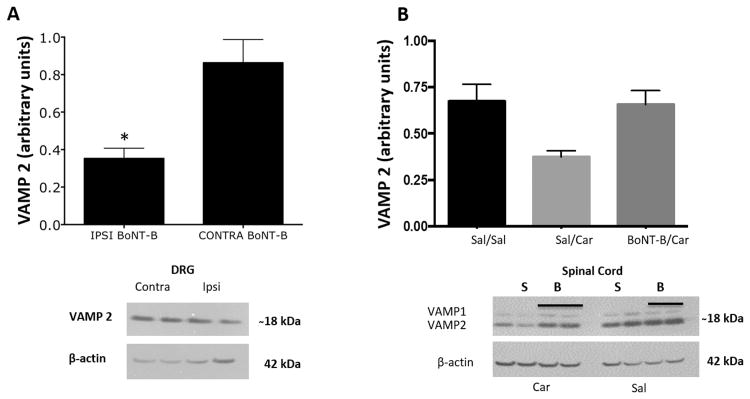
Figure 6. Effect of intraplantar BoNT-B pre-treatment on VAMP2 expression in dorsal root ganglion (DRG) and spinal cord dorsal horn.
A) Intraplantar BoNT-B injection (2 days prior) resulted in decreased VAMP2 in the ipsilateral pooled L5-L6 DRG compared to contralateral DRGs. Histogram depicts the relative levels of VAMP expression quantified by densitometric measurement. Representative Western blots are below each set of graphs. B) No difference was observed in the VAMP2 expression in spinal cord tissues following i.pl. BoNT-B. *p≤0.05 as compared to contra BoNT-B, paired t-tests. N=5. BoNT-B: botulinum toxin B, VAMP2: vesicle associated membrane protein 2.
Spinal cord quadrants, both ipsi- and contralateral to i.pl. BoNT-B injections were also assayed for VAMP 1 and 2. The means of the VAMP 1 and VAMP 2 for ipsi BoNT-B with carrageenan were 0.66+/-0.07 and 1.21+/- 0.12 respectively. In the animals that were pre-treated with saline the means were 0.37+/- 0.03 and 0.91+/-0.17. There was no difference for the VAMP 2 in the spinal cord (Fig. 6B), which is in accord with our previous findings (Marino et al., 2014). We have suggested that this absence of a significant effect is due to the small percentage of VAMP 2 from the entire dorsal spinal cord pool being affected by the restricted cutaneous afferents transporting the i.pl. BoNT delivery. Interestingly, VAMP 1 was significantly lower in the spinal tissue ipsilateral to saline/carrageenan as compared to tissue contralateral to the carrageenan at p=0.0471. Puzzlingly, VAMP 1 values ipsilateral to carrageenan were elevated back to control levels when BoNT-B was employed as a pre-treatment (p=0.428).
4. Discussion
4.1. Intraplantar BoNT-B blockade of primary afferent drive
Pre-treatment with i.pl. injection of BoNT-B significantly reduced carrageenan-induced mechanical hypersensitivity. These behavioral results were anticipated in light of previous studies in which pre-treatment with different serotypes of BoNT had no effect upon normal thresholds, but reduced or blocked sensitized pain states induced by carrageenan (Bach-Rojecky & Lackovic, 2005; Favre-Guilmard et al., 2009; Shin et al., 2013), formalin (Cui et al., 2004; Marino et al., 2014) and capsaicin (Chuang et al., 2007; Gazerani et al., 2009; Tugnoli et al., 2007) in a variety of human and animal models. The behavioral results associated with i.pl. BoNT are in accord with the effects observed here and in other studies showing a reduction in ipsilateral c-Fos expression in dorsal horn neurons otherwise induced by peripheral injection of inflammatory agents (Huang et al., 2011; Marino et al., 2014; Shin et al., 2013). In this study we extended these observations showing that dorsal horn markers of activity-driven spinal sensitization as observed following intraplantar carrageenan, notably phosphorylation of Akt (Choi et al., 2010; Shi et al., 2009; Sun et al., 2006; Xu et al., 2011) and the AMPA receptor subunit GluA1 were also reduced. Phosphorylation of GluA1 enhances probability of exocytotic membrane insertion of AMPAr (Esteban et al., 2003; Malinow & Malenka, 2002). Collectively these results can be explained by inhibition of primary afferent excitatory input into the spinal dorsal horn (Cui et al., 2004; Kitamura et al., 2009).
4.2. Lack of intraplantar BoNT-B effect on edema
It is clear that i.pl. BoNTs can have potent transmitter release inhibiting effects from peripheral peptidergic terminals as assessed by plasma extravasation evoked by local delivery of capsaicin (Chuang et al., 2007; Marino et al., 2014). Some clinical studies also have positive results showing that subcutaneous BoNT-A pre-treatment successfully reduced capsaicin or electrically induced neurogenic flare (Gazerani et al., 2009; Gazerani et al., 2006; Kramer et al., 2003; Tugnoli et al., 2007). In the present study, however, i.pl. BoNT-B was without effect on paw edema evoked by local carrageenan. Comparable results were noted with i.pl BoNT-A in rats (Bach-Rojecky et al., 2008; Favre-Guilmard et al., 2009). In a human inflammatory pain model consisting of ultraviolet-B irradiation of the leg, pre-treatment with BoNT-A 3 days before injury had no effect on superficial skin blood flow (Sycha et al., 2006). Pre-clinical studies have shown that genetic deletion of NaV 1.8 specific to nociceptors or loss of peptidergic c-fibres reduced inflammation induced pain behavior with no effect on edema and immune infiltration (Borbely et al., 2015; Chiu et al., 2013; Helyes et al., 2004). Further, carrageenan evoked edema/swelling remained unaffected in the rats that were neonatally treated with capsaicin (Gamillscheg et al., 1984). This indicates that swelling is not dependent upon the function of small TRPV1 (+) peptidergic afferents and probably the reason why intraplantar BoNT-B could not alter edema in the carrageenan-induced edema as compared to capsaicin induced plama extravasation which would be a more primary afferent fiber centered stimulus that might be more easily blocked by inhibition of peripheral neurotransmitter release. Importantly, the present studies demonstrate the dissociation between inflammation and the allodynia initiated by local inflammation. This also suggests that the change in afferent driven pain behavior does not necessarily co-vary with changes in the inflammatory state.
4.3. Dorsal horn actions of intraplantar BoNT-B
Previous work has suggested that peripherally delivered BoNTs diminish the excitatory spinal input generated by peripheral inflammation and these central effects account for the potent anti-hyperalgesic actions of peripheral BoNTs (Marino et al., 2014). Two lines of evidence support central actions of BoNTs after peripheral delivery: i) Direct activation of central afferent terminals by intrathecal delivery of capsaicin acting upon primary afferent TRPV1 receptors results in bilateral release of sP (as measured by NK1 internalization) and increased c-Fos expression in second order neurons, which is reduced in the ipsilateral dorsal horn by i.pl. BoNT (Marino et al., 2014). As the NK1 receptor is almost completely postsynaptic to the primary afferent (Littlewood et al., 1995), such injections imply a direct post synaptic activation. Ipsilateral block of this activation by i.pl. BoNT thus supports a post-synaptic action. Thus, it has been suggested that BoNTs transported in sensory afferents may undergo transynaptic/transcytotic movement to block SNARE function in second order dorsal horn neurons or other sensory afferents. ii) Injection of BoNT-B into the supraorbital region results in SNARE cleavage in trigeminal ganglion neurons that project to the meninges and blocks neurotransmitter release from meningeal afferents (Ramachandran et al., 2015).
Therefore, our first step was to determine whether BoNT pre-treatment interfered with binding of ligands to their receptors on second order neurons and/or to the subsequent phosphorylation of Akt or GluA1, markers for central sensitization, when the primary afferent was bypassed and activation was via intrathecal administration of NMDA or sP. Our working hypothesis was that these intracellular cascades would remain intact and that it would only be the SNARE-dependent insertion of GluA1 enriched AMPA receptors into the neuronal membrane that would be altered by BoNT, if the toxin were to be transported trans-synaptically into these neurons. Insertion of AMPAr into hippocampal neuronal membranes in vitro is blocked by direct exposure to botulinum toxin (Kanno et al., 2009; Schenk et al., 2003). Unexpectedly, phosphorylation of both Akt and GluA1 induced by spinal delivery of NMDA, but not sP, was substantially reduced by i.pl. BoNT-A pre-treatment. The most parsimonious explanation is that the sP effect is mediated predominantly by NK1 receptors, which are post synaptic to the primary afferent (Littlewood et al., 1995). In contrast, the i.t. NMDA effect could be mediated via presynaptic NMDA receptors present on the primary afferent fibers and to a lesser extent on the second order neuron. Several groups have demonstrated that in addition to a post-synaptic excitation of the 2nd order neuron, NMDA indeed evokes afferent terminal release of excitatory neurotransmitters (Chen et al., 2010; Liu et al., 1997; Liu et al., 1994), but see (Nazarian et al., 2008)). Activation of peripheral NMDA receptors on DRG neurons or satellite cells is sufficient to cause mechanical hyperalgesia in the behaving animal and sensitization of nociceptors (Ferrari et al., 2014; Parada et al., 2003; Zhou et al., 1996).
In conclusion, our data supports the growing literature for the therapeutic use of BoNTs for analgesia. So far, BoNT-A1 and BoNT-B are the only two clinically approved serotypes, and although they target different SNARES, the functional consequences do not differ from each other and are advantageous in restoring therapeutic responses in cases where one serotype develops resistance (Borodic et al., 1996; Greene et al., 1994). Inflammatory pain states such as myofascial pains and osteoarthritis entail enhanced primary afferent drive to central structures that underlies the initiation and maintenance of central sensitization (Gerwin 2012; Okun et al., 2012). The ability of peripheral BoNT to reduce spinal excitability and primary afferent neurotransmitter release may provide an important peripheral target for treatment of pain that can circumvents the central side effects of conventional analgesics. Although our present studies show that intraplantar BoNT-B does not alter local peripheral edema, BoNT is able to reverse the behavioral hypersensitivities associated with inflammation. These potent effects of peripherally delivered BoNT in altering central events and affecting only the hyperpathic component of the pain state presents itself as a potential therapeutic candidate that can be employed for treating inflammatory and other persistent pain conditions. The reduction in DRG VAMP and the effects on phosphorylation evoked by intrathecal NMDA confirm previous reports of a spinofugal transport of active toxin in sensory afferents to the DRG and beyond to their central terminals. Trans-synaptic transport and reduction of activity-driven membrane insertion of GluA1 enriched AMPA receptors remains to be determined.
Highlights.
Intraplantar BoNT-B reduced intraplantar carrageenan evoked allodynia, but not edema
Intraplantar BoNT-B inhibited carrageenan induced increase in pGluA1, pAkt and c-Fos expression.
Intraplantar BoNT-B inhibited NMDA, but not substance P evoked phosphorylation of GluA1 and Akt.
Acknowledgments
NIH DA02110, BoNT-B was gifted by Solstice Neurosciences. SS was supported by a Bogue Scholarship from UCL and IMI Europain. We would like to thank Shelly Malkmus, Joanne Steinauer for their technical assistance.
Abbreviations
- BoNT
botulinum toxin
- DRG
dorsal root ganglion
- HC
heavy chain
- i.pl.
intraplanatar
- i.t.
intrathecal
- LC
Light chain
- NMDA
N-methyl-D-aspartate
- PFA
paraformaldehyde
- SNARE
soluble N-methylaleimide-sensitive attachment protein receptor
- sP
substance P
- TRPV1
transient receptor potential vanilloid receptor
- TG
trigeminal ganglion
- TNC
trigeminal nucleus caudalis
- VAMP
vesicle associated membrane protein
Footnotes
Conflict of Interest: We declare that there is no conflict of financial interest with regard to our manuscript. Rimabotulinumtoxin B, Myobloc ® was provided by Solstice Neurosciences.
References
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-Rojecky L, Dominis M, Lackovic Z. Lack of anti-inflammatory effect of botulinum toxin type A in experimental models of inflammation. Fundam Clin Pharmacol. 2008;22:503–509. doi: 10.1111/j.1472-8206.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- Bach-Rojecky L, Lackovic Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005;46:201–208. [PubMed] [Google Scholar]
- Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94:234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Borbely E, Botz B, Bolcskei K, Kenyer T, Kereskai L, Kiss T, Szolcsanyi J, Pinter E, Csepregi JZ, Mocsai A, Helyes Z. Capsaicin-sensitive sensory nerves exert complex regulatory functions in the serum-transfer mouse model of autoimmune arthritis. Brain Behav Immun. 2015;45:50–59. doi: 10.1016/j.bbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodic G, Johnson E, Goodnough M, Schantz E. Botulinum toxin therapy, immunologic resistance, and problems with available materials. Neurology. 1996;46:26–29. doi: 10.1212/wnl.46.1.26. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 2010;166:924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck WJ, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Yoshimura N, Wu M, Huang CC, Chiang PH, Tyagi P, Chancellor MB. Intraprostatic capsaicin injection as a novel model for nonbacterial prostatitis and effects of botulinum toxin A. Eur Urol. 2007;51:1119–1127. doi: 10.1016/j.eururo.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Elferink LA, Trimble WS, Scheller RH. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989;264:11061–11064. [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Favre-Guilmard C, Auguet M, Chabrier PE. Different antinociceptive effects of botulinum toxin type A in inflammatory and peripheral polyneuropathic rat models. Eur J Pharmacol. 2009;617:48–53. doi: 10.1016/j.ejphar.2009.06.047. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Lotufo CM, Araldi D, Rodrigues MA, Macedo LP, Ferreira SH, Parada CA. Inflammatory sensitization of nociceptors depends on activation of NMDA receptors in DRG satellite cells. Proc Natl Acad Sci U S A. 2014;111:18363–18368. doi: 10.1073/pnas.1420601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic B, Matak I, Bach-Rojecky L, Lackovic Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS One. 2012;7:e29803. doi: 10.1371/journal.pone.0029803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamillscheg A, Holzer P, Donnerer J, Lembeck F. Effect of neonatal treatment with capsaicin on carrageenan-induced paw oedema in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1984;326:340–342. doi: 10.1007/BF00501439. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Pedersen NS, Staahl C, Drewes AM, Arendt-Nielsen L. Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain. 2009;141:60–69. doi: 10.1016/j.pain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122:315–325. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Gerwin R. Botulinum toxin treatment of myofascial pain: a critical review of the literature. Curr Pain Headache Rep. 2012;16:413–422. doi: 10.1007/s11916-012-0287-6. [DOI] [PubMed] [Google Scholar]
- Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994;9:213–217. doi: 10.1002/mds.870090216. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Szabo A, Nemeth J, Jakab B, Pinter E, Banvolgyi A, Kereskai L, Keri G, Szolcsanyi J. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50:1677–1685. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Huang PP, Khan I, Suhail MS, Malkmus S, Yaksh TL. Spinal botulinum neurotoxin B: effects on afferent transmitter release and nociceptive processing. PLoS One. 2011;6:e19126. doi: 10.1371/journal.pone.0019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427–446. doi: 10.1016/s0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Yuzaki M. A mechanism underlying AMPA receptor trafficking during cerebellar long-term potentiation. Proc Natl Acad Sci U S A. 2005;102:17846–17851. doi: 10.1073/pnas.0508910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Yaguchi T, Nagata T, Tanaka A, Nishizaki T. DCP-LA stimulates AMPA receptor exocytosis through CaMKII activation due to PP-1 inhibition. J Cell Physiol. 2009;221:183–188. doi: 10.1002/jcp.21838. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee GW, Kim MJ, Yang KY, Kim ST, Bae YC, Ahn DK. Antinociceptive Effects of Transcytosed Botulinum Neurotoxin Type A on Trigeminal Nociception in Rats. Korean J Physiol Pharmacol. 2015;19:349–355. doi: 10.4196/kjpp.2015.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Matsuka Y, Spigelman I, Ishihara Y, Yamamoto Y, Sonoyama W, Kamioka H, Yamashiro T, Kuboki T, Oguma K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience. 2009;159:1422–1429. doi: 10.1016/j.neuroscience.2009.01.066. [DOI] [PubMed] [Google Scholar]
- Kramer HH, Angerer C, Erbguth F, Schmelz M, Birklein F. Botulinum Toxin A reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J Neurol. 2003;250:188–193. doi: 10.1007/s00415-003-0971-x. [DOI] [PubMed] [Google Scholar]
- Lawrence GW, Ovsepian SV, Wang J, Aoki KR, Dolly JO. Extravesicular intraneuronal migration of internalized botulinum neurotoxins without detectable inhibition of distal neurotransmission. Biochem J. 2012;441:443–452. doi: 10.1042/BJ20111117. [DOI] [PubMed] [Google Scholar]
- Littlewood NK, Todd AJ, Spike RC, Watt C, Shehab SA. The types of neuron in spinal dorsal horn which possess neurokinin-1 receptors. Neuroscience. 1995;66:597–608. doi: 10.1016/0306-4522(95)00039-l. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci U S A. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Terashima T, Steinauer JJ, Eddinger KA, Yaksh TL, Xu Q. Botulinum toxin B in the sensory afferent: Transmitter release, spinal activation, and pain behavior. Pain. 2014;155:674–684. doi: 10.1016/j.pain.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Molgo J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 2005;5:274–279. doi: 10.1016/j.coph.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. Spinal N-methyl-D-aspartate receptors and nociception-evoked release of primary afferent substance P. Neuroscience. 2008;152:119–127. doi: 10.1016/j.neuroscience.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Vivancos GG, Tambeli CH, Cunha FQ, Ferreira SH. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci U S A. 2003;100:2923–2928. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett S, Yaksh TL, Ramachandran R. Current Status and Future Directions of Botulinum Neurotoxins for Targeting Pain Processing. Toxins (Basel) 2015;7:4519–4563. doi: 10.3390/toxins7114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Lam C, Yaksh TL. Botulinum toxin in migraine: Role of transport in trigemino-somatic and trigemino-vascular afferents. Neurobiol Dis. 2015;79:111–122. doi: 10.1016/j.nbd.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: mechanisms of action. Br J Pharmacol. 2014;171:4177–4192. doi: 10.1111/bph.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31:15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk U, Verderio C, Benfenati F, Matteoli M. Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO J. 2003;22:558–568. doi: 10.1093/emboj/cdg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de LP, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Huang P, Mulder J, Ceccatelli S, Hokfelt T. Expression of p-Akt in sensory neurons and spinal cord after peripheral nerve injury. Neurosignals. 2009;17:203–212. doi: 10.1159/000210400. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, Kuroi T, Ebine T, Koizumi K, Suzuki N. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis. 2012;48:367–378. doi: 10.1016/j.nbd.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Shin MC, Yukihira T, Ito Y, Akaike N. Antinociceptive effects of A1 and A2 type botulinum toxins on carrageenan-induced hyperalgesia in rat. Toxicon. 2013;64:12–19. doi: 10.1016/j.toxicon.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain. 2006;120:86–96. doi: 10.1016/j.pain.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sycha T, Samal D, Chizh B, Lehr S, Gustorff B, Schnider P, Auff E. A lack of antinociceptive or antiinflammatory effect of botulinum toxin A in an inflammatory human pain model. Anesth Analg. 2006;102:509–516. doi: 10.1213/01.ane.0000194447.46763.73. [DOI] [PubMed] [Google Scholar]
- Tugnoli V, Capone JG, Eleopra R, Quatrale R, Sensi M, Gastaldo E, Tola MR, Geppetti P. Botulinum toxin type A reduces capsaicin-evoked pain and neurogenic vasodilatation in human skin. Pain. 2007;130:76–83. doi: 10.1016/j.pain.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Xu Q, Fitzsimmons B, Steinauer J, O'Neill A, Newton AC, Hua XY, Yaksh TL. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J Neurosci. 2011;31:2113–2124. doi: 10.1523/JNEUROSCI.2139-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7:895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]