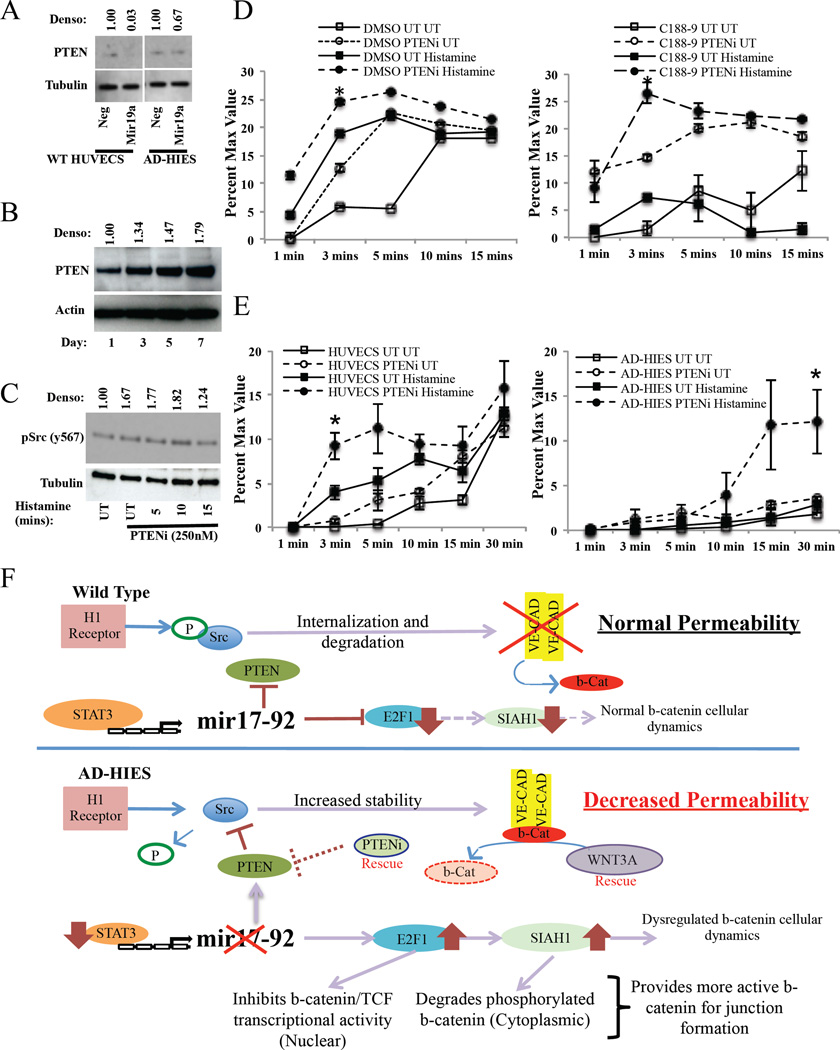
Figure 6. PTEN inhibition restores STAT3-inhibited or mutated HUVECs permeability.
(A) PTEN expression in wild-type and AD-HIES HUVECs following transfection with a mir19 mimic. (B) PTEN expression in STAT3 inhibited wild-type HUVECs over 7 days. (C) Expression of phosphorylated Src in PTEN inhibitor pre-treated HUVECs (1h) that were subsequently exposed to histamine (100µM). (D–E) In vitro permeability assay of (D) DMSO or C188–9 treated HUVECs or (E) wild-type and AD-HIES HUVECs treated with a PTEN inhibitor (1h) and subsequently exposed to histamine (100µM). (F) Model of STAT3-regulation of vascular permeability in wild type and AD-HIES HUVECs. In wild type cells, histamine treatment leads to Src phosphorylation resulting in the dissociation of β-catenin and the internalization and degradation of VE-cadherin. Under conditions of normal STAT3 signaling, STAT3 induces expression of the mir17–92 microRNA cluster that suppresses PTEN, E2F1 and SIAH1 levels. This allows for the normal regulation of Src signaling and normal distribution of β-catenin cellular dynamics and thus, directly, regulates the amount of VE-cadherin and β-catenin. In STAT3-mutated cells, histamine treatment leads to no Src phosphorylation, due to decreased expression of mir17–92 resulting in increased PTEN. This prevents the internalization and degradation of VE-cadherin, ultimately increasing its expression. Mir17–92 deficiency also allows for increased E2F1 and SIAH1. This will degrade any phosphorylated β-catenin in the cytoplasm and inhibits nuclear translocation of non-phosphorylated cytoplasmic β-catenin. This provides more non-phosphorylated β-catenin to form adherin junctions, leading to decreased vascular permeability.