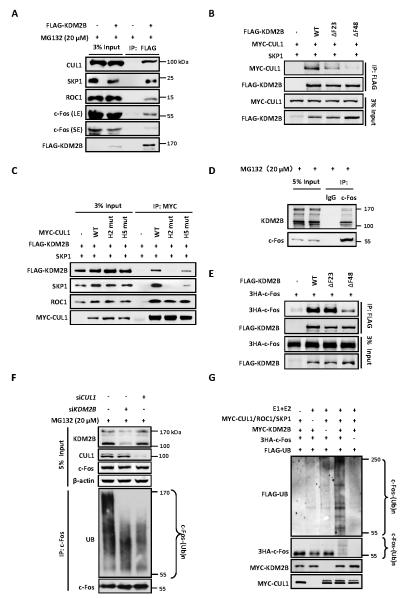
Figure 2. c-Fos is a substrate of SCFKDM2B/FBXL10 E3 ubiquitin ligase.
(A) Ectopically expressed KDM2B binds to endogenous CUL1/SKP1/ROC1 E3 ligase. HEK293T cells were transfected with plasmid expressing FLAG-tagged KDM2B and treated with 20 μM MG132 for 6 h. The interactions between KDM2B and endogenous CUL1, SKP1, ROC1 and c-Fos proteins were determined by Co-IP assay. LE and SE refer to longer exposure and short exposure, respectively.
(B) The F-box domain of KDM2B is essential for its binding to CUL1. HEK293T cells were transfected with plasmids expressing indicated proteins. The interactions between WT or mutant KDM2B and CUL1 were determined by Co-IP assay.
(C) The H2 and H5 helixes of CUL1 are essential for its binding to KDM2B. HEK293T cells were transfected with plasmids expressing indicated proteins. The interactions between KDM2B and WT or mutant CUL1 were determined by Co-IP assay.
(D) Endogenous interaction between c-Fos and KDM2B two isoforms determined by Co-IP. HEK293 cells were treated with 20 μM MG132 for 6 h and cell lysates were used for IP with IgG or c-Fos antibody.
(E) The C-terminal of F-box domain in KDM2B is required for its binding to c-Fos. HEK293T cells were transfected with plasmids expressing indicated proteins. The interactions between WT or mutant KDM2B and c-Fos were determined by Co-IP.
(F) Knock down of either KDM2B or CUL1 decreases c-Fos ubiquitylation in vivo. HEK293 cells were transfected with siRNA oligonucleotides targeting indicated genes and treated with 20 μM MG132 for 6 h. Endogenous c-Fos was immunoprecipitated and immunoblotted with an antibody specific against ubiquitin.
(G) In vitro ubiquitylation of c-Fos by SCFKDM2B E3 ubiquitin ligase. Purified c-Fos protein was incubated with immunopurified E3 CUL1 complex and KDM2B individually or in combination in the presence or absence of E1, E2, ATP and Flag-ubiquitin in vitro for 1 h. The reaction mixture was resolved by SDS-PAGE and blotted using antibodies (from top to bottom panels) recognizing FLAG, HA, and MYC.