Abstract
Cardiovascular disease is a leading cause of death worldwide. Since adult cardiac cells are limited in their proliferation, cardiac tissue with dead or damaged cardiac cells downstream of the occluded vessel does not regenerate after myocardial infarction. The cardiac tissue is then replaced with nonfunctional fibrotic scar tissue rather than new cardiac cells, which leaves the heart weak. The limited proliferation ability of host cardiac cells has motivated investigators to research the potential cardiac regenerative ability of stem cells. Considerable progress has been made in this endeavor. However, the optimum type of stem cells along with the most suitable matrix-material and cellular microenvironmental cues are yet to be identified or agreed upon. This review presents an overview of various types of biofunctional materials and biomaterial matrices, which in combination with stem cells, have shown promises for cardiac tissue replacement and reinforcement. Engineered biomaterials also have applications in cardiac tissue engineering, in which tissue constructs are developed in vitro by combining stem cells and biomaterial scaffolds for drug screening or eventual implantation. This review highlights the benefits of using biomaterials in conjunction with stem cells to repair damaged myocardium and give a brief description of the properties of these biomaterials that make them such valuable tools to the field.
Keywords: angiogenesis, hydrogel, myocardial infarction, nanomedicine, stem cells
Graphical Abstract
1. Introduction
Myocardial infarction (MI) is one of the main causes of morbidity and mortality around the world.[1] Each year around one million deaths occur due to heart failure in the United States alone.[2] MI results from the obstruction of cardiac vessels which halts blood flow to part of the heart causing damage to that portion of the myocardium. Blood flow can be restored in the occluded tissues by balloon angioplasty, stenting, and cardiac bypass surgeries; however, the damaged cardiac tissue does not self-regenerate and the heart remains weak. Recent developments in stem cell research aim to restore injured myocardium by regenerative medicine and tissue engineering methods.
Adult cardiomyocytes are terminally differentiated cells that have a minimal proliferation capacity and that cannot be extracted in sufficient numbers. A potential alternative to cardiomyocytes for cardiac repair is stem cells—a self-renewable and differentiable cell source that has provoked increased cardiac function following cell-based therapy.[3] Some types of stem cells, such as mesenchymal stem cells (MSCs) and cardiac stem cells (CSCs), have made it to human trials. Recently, attention has turned toward the potential therapeutic effects of partially differentiated stem cells such as adipose-derived adult stem cells and endothelial progenitor cells. Endothelial progenitor cells were shown to directly trigger pro-angiogenic factors and healing responses that may be of potential use for tissue engineering and regenerative medicine. Embryonic stem cells (ESCs) are another source of stem cells that have engendered propitious results in cardiac regeneration. ESCs are pluripotent, are easily handled and proliferated, and were shown to differentiate into cardiomyocytes.[4] Animal studies have demonstrated some repairs in damaged heart muscle using this cell line. Despite positive results from ESCs, numerous ethical controversies have driven research away from them. A solution to these concerns may be found in somatic cells that have been “reprogrammed” and found to mimic ESCs engineered induced pluripotent stem cells (iPSCs).[5] They are among the most recent ESC alternative sources under investigation in animal models. More effective cardiac therapy for any of the above stem cells has been achieved through the careful regulation of stem cell environment in conjunction with the addition of growth factors and chemokines.
Regenerative medicine and tissue engineering techniques are at the center of the most promising “nextgeneration” therapies for MI.[3] Regenerative medicine is intended to repair the damaged cardiac tissue in vivo by injecting cells directly into the heart. Several delivery routes have been proposed, such as intracoronary, intravenous, intramyocardial, retrograde sinus, and intrapericardial routes. None of the stem cell delivery routes has yet been proclaimed to be superior to the others and each one has individually shown some advantages. A vehicle that scientists have used in order to increase the efficacy of stem cell injections in regenerative medicine is injectable hydrogels. Alternatively, tissue engineering combines expertise in cell biology, engineering, and materials in order to grow a cardiac tissue in vitro. This tissue construct is engineered to mimic the behavior of human heart tissue and is later transplanted into a patient’s heart to improve or replace nonfunctional cells. Undifferentiated stem cells placed within cardiac constructs have been tested in MI animal models and have shown to improve survival rate, decrease the area of the damaged myocardium, and increase overall cardiac function. In these instances, stem cells differentiated into cardiomyocytes in vivo, and thus the differentiation process was uncontrolled and sometimes resulted in the development of teratomas. In further studies, scientists have differentiated stem cells in vitro prior to implantation of the cardiac construct, which offered improved results. Among the most successful methodologies in tissue engineering are the use of cell sheets and porous scaffolds. While neither regenerative medicine nor tissue engineering methods are sufficient as independent solutions for cardiac cell repair following MI, these approaches are advancing fast which demonstrates a strong potential for a stand-alone solution.
Recent advances are looking toward the refinement of current techniques and the minimization of complications. For example, the differentiation of stem cells into the right cell lineage as well as the preservation of the genetic footprint are sometimes problematic and can be hindered by the development of teratomas.[6] Investigations have revealed that the source of stem cells is an important factor that affects cell differentiation. In addition, only a few of biomaterials used in regenerative medicine and tissue engineering have made it to human trials and improvements are necessary in terms of their mechanical, biological, and physical properties to provide optimal conditions for stem cell growth, differentiation, and retention.[7]
2. Stem Cells to Heal Damaged Heart
Stem cells are undifferentiated cells that exist in various regions of the body and have two main roles. The first is growth for embryos and tissue maintenance and the second is regeneration and repair for adults.[3] There are several properties that stem cell types must exhibit in order to be selected as candidates for cardiac therapy. Primarily, they should be able to increase heart function by differentiating into cardiomyocytes when placed in a suitable environment and/or through indirect paracrine effects. The ability of stem cells to differentiate depends on their potency: pluripotent, multipotent, totipotent, or unipotent. The environment in which stem cells are cultured or delivered can facilitate the process of their differentiation into cardiomyocytes. Second, the cells must be relatively easy to isolate and maintain, and they should also have the ability to proliferate in sufficient numbers. In addition, they should be able to survive harvesting, delivery and the harsh microenvironment of the infarcted myocardium. Finally, stem cells should cause a low immunogenic response lest the benefits of the cells be completely annulled by the damage of an immunological response.[8]
Since cardiac tissue engineering and regenerative medicine requires a large quantity of cells, terminally differentiated adult cardiomyocytes which have a low proliferative capacity are unsuitable for these applications. Stem cells, on the other hand, can appropriately differentiate into cardiomyocytes and proliferate enough to meet the quantity requirements. Due to the relatively easy accessibility of resident stem cells, an ideal method would be to use cardiac-like cells derived from this type of stem cell.[9] The types of stem cells that have been used widely in animal models for cardiac repair are embryonic stem cells, induced pluripotent stem cells and stem cells from various adult tissues.[10]
2.1. Types of Stem Cells
ESCs fulfill most of the requirements for stem cell based cardiac therapy including clonality, self-renewal and pluripotency.[3,10] ESCs have shown a clear ability to differentiate into functional cardiomyocytes and endothelial cells, therefore, they have the potential to reproduce several elements of damaged heart.[8] Cardiomyocytes derived from human ESCs have been tested on animal models and have been shown to survive after their transplantation, partly remodel the infarcted area, and positively improve contractile function.[11] Recently, researchers have been able to “reprogram” somatic cells to get them to an ES cell-like state by transduction of defined transcription factors.[3,12] These “engineered” ESCs called iPSCs have been shown to differentiate into functional cardiomyocytes.[13,14] The iPSCs present an appropriate alternative to human ESCs by surpassing ESCs ethical concerns and providing an ideal autologous cell source, which reduces the chances of an immunological response.[5,15] Also, the use of iPSCs bypasses the need for invasive surgery because of the wide availability and accessibility of cells eligible for suitable reprogramming.[16] Although iPSCs have not yet reached a human trials phase, they have shown promising survival results in the infarcted myocardium and marginal renewal of heart function in animal models that are comparable to other cell sources. However, much work remains to be done in order to enhance their engraftment, survival rate, and therapeutic efficiency.[16]
Adult stem cells are another option that scientists have extensively studied. These cells are in several locations throughout the human body.[17] For cardiac purposes, scientists have used cells from several sources such as the heart, bone marrow, umbilical cord, placenta, and adipose tissue. For example, hematopoietic stem cells can be found in umbilical cords, placenta, and bone marrow.[3] MSCs can be harvested from femoral heads, endometrium, bone marrow and adipose tissue.[18] Due to their ease of accessibility through noninvasive surgery and their low immunogenicity, MSCs are good candidates for allogeneic stem cell therapies. In addition, they have a high proliferative potential and can be grown extensively in vitro, making them an attractive option for scientists.[17] Many studies on animal models have shown that the injection of MSCs is beneficial in the case of cardiac injuries and diseases.[19] The cells have been shown to differentiate into beating heart cells in vitro, to increase vascularization density when transplanted into ischemic murine model, and to prevent scar expansion.[20,21] MSC human therapy trials have recently started with initial success.[22] Among the different origins of MSCs, adipose-derived stem cells (ASCs) have shown great promise for an autologous source due to their large availability and relative ease of isolation through liposuction.[3,19] Around a decade ago, CSCs were found in the heart using negative c-kit,[23] Sca-1,[24] isl1[25] and cardiosphere-forming cells[26] stem cell markers. Since then, scientists have investigated their use in regenerative medicine using methods such as cardiosphere-forming technology to isolate CSCs from heart tissue. One example is the SCIPIO human trial that is being conducted with autologous c-kit+ CSCs for the treatment of MI. Results have revealed that the intracoronary delivery of these cells is safe, improves LV systolic function and reduces the size of the ischemic tissue. However, surviving and engrafted cells are low in number.[27,28] Along with CSCs, another important adult cell type that needs to be considered is endothelial progenitor cells (EPCs). These cells are defined as circulating cells that express a variety of cell surface markers similar to those expressed by vascular endothelial cells, adhere to endothelium at sites of hypoxia/ischemia, mediate tissue-protective effects, and participate in new vessel formation.[29] In an experimentally induced MI, neoangiogenesis triggered by EPCs resulted in decreased apoptosis of hypertrophic myocytes in the peri-infarct region, longterm salvage and survival of viable myocardium, reduction in collagen deposition, and sustained improvement in cardiac function.[30]
Each of the sources of cells previously mentioned have their own drawbacks that scientists are striving to overcome. CSCs and EPCs do not pose an ideal solution due to their scarcity.[31] Isolation of adult stem cells remains a problem since it usually results in a heterogeneous population of cells, which requires the use of cell surface markers to isolate the required stem cell types. The use of ESCs is significantly hindered by ethical issues as well as risks of teratoma formation.[12,32] Also, more research needs to be conducted to study iPSC behavior in order to minimize the possibility of teratoma formation and optimize therapeutic benefit in damaged myocardium. Scientists are working hard to resolve these issues to determine the ideal source for cardiac tissue engineering and regenerative medicine. Many studies are being conducted to better define the intrinsic nature of the adult stem cell types mentioned above as well as the mechanism behind some of the noted differentiation, transdifferentiation and engraftment contributing to the improvement of cardiac function. Despite the fact that stem cell grafting and retention has remained low, results look promising.
2.2. Regenerative Medicine Cell Delivery Routes
Regenerative medicine induces myocardial repair at the site of injury in vivo and prevents ventricular dilatation after MI through an injection of stem cells. Successful delivery is crucial for an ideal stem cell therapy into the cardiac tissue. There are five main delivery routes: intracoronary, intravenous, intramyocardial, retrograde sinus and intrapericardial.[33] The intracoronary infusion technique involves cell fusion through the central lumen of a balloon catheter positioned in the coronary artery.[34] This route limits risks of systemic administration, as the delivery concentration is higher in ischemic and border zone regions. It is generally used for patients who have electrocardiographic ST segment elevation MI or patients with chronic myocardial ischemia.[35] The simplest delivery route is intravenous delivery. It has the advantage of being noninvasive and is often referred to as one of the safest delivery methods.[36] It is usually done through a peripheral or central venous catheter.[37] However, a high number of exposed cells may end up in other organs and only a small amount will reach the target area, which reduces the efficiency of this technique.[38–40] Furthermore, patients with occluded arteries usually cannot benefit from an intravenous injection. Another method is the intramyocardial injection. This delivery method consists of the direct injection of stem cells into the myocardium via a transepicardial or transendocardial injection. It is generally used for patients with an ischemic cardiomyopathy. The exact location of the infarcted tissue can be identified using echocardiography or nuclear imaging.[41] Transepicardial injection is performed using a needle-syringe while directly visualizing the heart.[42] However, the operation is risky due to the need for open-heart surgery.[43] The transendocardial approach does not require surgery, but it provides less visibility because it is performed in a percutaneous way.[44] Catheters are passed from peripheral vessels into the left ventricular cavity.[42] Electromechanical mapping systems can be used to increase visibility during the procedure. The drawbacks of both techniques include increased risks of perforation, embolization and cardiac arrhythmias.[45] The retrograde coronary sinus delivery consists of placing a balloon infusion catheter after catheter placement into the coronary sinus. The balloon is then inflated and cells are delivered at pressures of 50–60 mm Hg maximum, which are higher than the coronary sinus pressure.[46] This emerging technique may be efficacious and is considered safe.[47] Recent studies have shown that injection using this method delivers cells with a better homogeneity across the heart.[48] The last injection method is the intrapericardial injection. One of its advantages is a relatively high number of deposited cells. However, the movement of cells across the visceral pericardium is essential. Little is known about the efficiency of this technique, as it is the least researched. To date, there is no specific route that has been proclaimed to be the finest technique for cellular therapy. Table 1 summarizes the prospects and challenges of different cell delivery strategies to maximize the stem cell based therapeutic strategies for cardiac tissue repair and regeneration.
Table 1.
Advantages and drawbacks of different types of stem cells, delivery methods and fabricated biomaterials used.
| Stem cell type | Advantages | Drawbacks | Ref. |
|---|---|---|---|
| Embryonic | Clonality, self-renewal, pluripotency |
Ethical concern |
[3,10,12,32] |
| Very sensitive to temperature and pH changes |
|||
|
Bone marrow derived adult stem cells |
No ethical issues; widely investigated under pre-clinical and clinical settings |
Access requires invasive surgery |
[139] |
| Isolation is required due to heterogeneous cell population |
|||
|
Adipose derived adult stem cells |
Less invasive and painful surgery |
Isolation is required due to heterogeneous cell population |
[3,19,139] |
| Induced pluripotent | Alternative to embryonic cells |
Probability of forming teratoma |
[5,25] |
| Availability of producing large amounts of patient-specific cells |
|||
| Endothelial progenitor | Production of large amounts of growth factors and cytokines |
Rare population | [31,140] |
| Delivery methods | Advantages | Drawbacks | Ref. |
| Intracoronary | No risk of systemic delivery |
Low cell delivery | [35] |
| Direct delivery | |||
| Intravenous | Not invasive | Cells can be isolated in lung, liver or spleen |
[36,38,141] |
| Intramyocardial | Direct delivery | Perforation risk | [43] |
| Intrapericardial | Large number of cells delivered |
Visceral pericardium transmigration required |
[40] |
| Retrograde coronary sinus | Homogeneous cell delivery | Endothelial wall transmigration required |
[48] |
| Biomaterials | Advantages | Possible concerns | Ref. |
| Cell sheets | Long-term survival and growth rate after implementation |
Retention of implant at transplant site; integration to host tissue |
[79,81] |
| Injectable hydrogels | Enhance stem cell retention and survivability upon injection |
Suitable rheological properties of the injected materials |
[142] |
| Porous scaffolds | Increased Vascularization | Biodegradation properties | [92] |
3. Mode of Therapeutic Actions: Knowledge So Far
There have been several studies that show positive remodeling of damaged myocardium after stem cell therapy. However, some ambiguity still remains on the mechanisms of engraftment of stem cells and their subsequent beneficial effects to the ischemic cardiac zone. The original theory that scientists proposed was that the stem cells delivered to the myocardium differentiate into new cardiomyocytes to produce the positive remodeling of the damaged tissue. However, due to scientific evidence that will be discussed in the following section, several scientists now believe that paracrine signaling between the delivered stem cells and resident stem cells are responsible for those effects.
3.1. Paracrine Signaling
Extracellular growth factors, cytokines and ligands constitute parts of signaling pathways at the center of cardiomyocyte differentiation, development, growth, function and metabolism. Low engrafted cell survival and proliferation with a relatively high outcome in regeneration suggests the involvement of paracrine factors secreted by the implanted cells. Stem cell homing factors such as growth factors, chemokines and cytokines are believed to improve cardiac function through neovascularization, angiogenesis, decreased apoptosis, decreased fibrosis, increased cell proliferation, as well as stem cell mobilization, differentiation and migration to the infarct zone. Significant effort is being put toward both understanding the mechanisms by which these biological molecules produce these positive results as well as delivering these molecules using different techniques. These investigations are indicating that these biological molecules could potentially be used in regenerative medicine and tissue engineering to aid stem cells grafting and retention in the heart.
Cytokines play an important role in cell differentiation, proliferation and apoptosis. They constitute a wide range of proteins that are involved in the communication and interactions between cells. Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are cytokines that are released shortly after acute MI.[31] The sustained presence of cytokines leads to the modification in myocyte phenotype and activation of matrix metalloproteinases, which modifies the interstitial matrix and further augments the remodeling processes.[31] Cytokines have the ability to trigger not only beneficial pathways to cellular regeneration such as restoration of function and angiogenesis, but also detrimental pathways such as apoptosis, chronic dilatation and stroke.[49] Chemokines are chemotactic cytokines that play an important role in several processes, including angiogenesis.[50,51] A stem cell homing factor that has recently received considerable attention is the stromal-derived factor (SDF)-1-α.[52] This chemokine has been shown to increase transcoronary migration and homing of stem cells to the ischemic myocardium,[53,54] angiogenesis, cell survival,[52,55] and improve ventricular function.[56] Recent research has shown that these positive results are due to the local release of SDF-1-α by MSCs that leads to the recruitment of CXCR4+ cardiac progenitor cells.[57] Upon the SDF-1-α:CXCR4 binding an antiapoptotic effect mediated by the phosphoinositide 3-kinase/Akt signaling pathway was shown. Lastly, the signaling associated with this pathway was shown to not cause cardiomyocyte hypertrophy, therefore, eliminating hypertrophy as a cause of positive cardiac remodeling.
In order to increase the efficacy of these paracrine factors and to take advantage of other delivery routes, scientists have recently explored delivery systems such as microparticles.[58,59] These microparticles overcome several drawbacks associated with direct injection of the paracrine factors including protein instability and short circulation protein half-life after injection. Specifically, the PLGA microparticles used by Formiga et al. were shown to provide sustained delivery of VEGF165 for more than one month and to increase angiogenesis and arteriogenesis. On the other hand, the VEGF solution and empty microparticles did not show these results after one month. Using particles to increase the clinical viability of paracrine factors shows great potential for cardiac regeneration. However, degradation, size, protein half-life, and release kinetics are all important factors that need to be carefully considered when deciding on a delivery technique.
Although researchers have some cues for reaching effective stem cell therapies for the regeneration of the heart, there are still many unknown mechanisms that are currently under investigation. Concentration on the synergistic effects of growth factors, cytokines and adequate stem cells reveal complex questions but promising results.
3.2. Differentiation of Stem Cells
Several scientists are studying ways to differentiate stem cells into cardiomyocytes. Successful methods are characterized by the expression of the correct genetic footprints and prevention of tumor cell development. Various techniques are being developed to ensure that the correct genetic footprint is maintained. One example is seeding the cells on scaffolds, which is expected to prevent the loss of genetic markers of differentiated cardiomyocytes especially after implantation into the host cardiac tissue.[60] Furthermore, it is important that the developed methods be scalable to allow the transition from experimental to clinical applications.[61]
One source of stem cells that has shown capability to be differentiated into cardiac muscle lineages is bone marrow.[62] Bone marrow stem cells have been applied to various applications such as cardiac patch tissue engineering.[20] According to Fukuda et al., mouse bone-marrow stem cells differentiated into a cardiac cell line demonstrate cardiomyocyte genes. In addition, spontaneous beating and pacemaker characteristics that mimic native ventricular-myocyte and sinus-node functions have also been noted. Nevertheless, this cell source also presents some challenges, such as the tendency to differentiate into bone and cartilage cells. Another significant problem seen with this cell line is that the delivered action potentials have smaller values than those found in native human myocardium.
Several factors have been found to affect the differentiation of stem cells into cardiomyocytes. The source of the stem cells greatly affects the lineage they will differentiate into, as revealed by Tompkins et al. through their discussion on stem cell “memories”.[60] For example, human cardiomyocytes differentiated from cardiac derived iPSCs were more easily obtained than those differentiated from fibroblast derived iPSCs. Scientists have also found success deriving cardiac cells from bone marrow derived MSCs.[63] Another factor that can also affect the differentiation of stem cells from various sources into cardiomyocytes is the addition of external molecules. Inhibitors can also be used to promote differentiation into cardiac cell lineage. For instance, the inhibitor trichostatin A has been shown to promote embryonic stem cell differentiation into cardiomyocytes and to increase the physical maturation of these cells.[60]
However, the differentiation of stem cells into cardiomyocytes has also resulted in teratoma development. The source of the developed cancer cells is mainly the stem cells that remain in their undifferentiated state in the culture. Several methods have been proposed to remove undifferentiated stem cells and prevent cancer cell growth in the differentiated cardiomyocytes.[64] Differentiated cardiomyocytes can be identified and separated from undifferentiated stem cells or those that have differentiated into other lineages by flow cytometry, micro-dissection, genetic selection, and buoyancy measurements.[60] However, some of these strategies themselves induce mutations and teratomas in the cell cultures. Therefore, future work needs to be done on how to negate this teratoma formation in order for stem cell therapy to become a viable widespread treatment option.
4. Boosting Stem Cell Therapy with Engineered Biomaterials
Different methodologies have been used to deliver stem cells to damaged myocardium including cell sheets, injectable hydrogels, porous scaffolds, cell-surface engineering, and microcapsules. Both natural and synthetic polymers have been used to make such delivery devices with each type of material providing its own benefits and challenges. This section will focus on the current research reported using each of these methods. Also, the current challenges of each method will be discussed along with strategies scientists are using to overcome these challenges to produce the best therapeutic benefit.
Natural polymers have several properties that make them ideal for tissue engineering and regenerative medicine. One of the most notable and unique characteristics is their bioactivity through providing signals to cells. Examples of natural polymers used in cardiac tissue engineering are collagen,[65] gelatin,[66] hyaluronan,[67] fibrin,[68] chitosan,[69] alginate[70] and Matrigel.[71] Fibrin, collagen I, and Matrigel, are commercially purchased polymers that can be used as hydrogels in cardiac patch tissue engineering with results showing improved cardiac function as well as increased vascularization of the grafted tissues.[72] One study discovered that human ESCs seeded on fibrin-based matrices formed highly functionalized cardiac tissues and improved results were seen in 3D gels compared to 2D gels. The properties compared were sarcomere length and presence of certain genes for cardiac contractility.[73] Natural polymers can also be used for an electrospun scaffold, as seen when a hemoglobin/gelatin/fibrinogen (Hb/gel/fib) nanofiberous construct was used to study MSC differentiation into cardiomyocytes.[74] Immunocytochemical assays for cardiac marker protein actinin showed that the MSCs did indeed begin to differentiate toward a cardiomyocyte lineage. The cells also began to exhibit morphological characteristics indicative of cardiomyocytes. However, in general, natural polymers suffer from poor mechanical properties and are prone to generating immune response from the host.[75] Nevertheless, if these mechanical problems can be overcome, the bioactivity of the natural polymers can prove to be a valuable tool in controlling stem cell behavior.
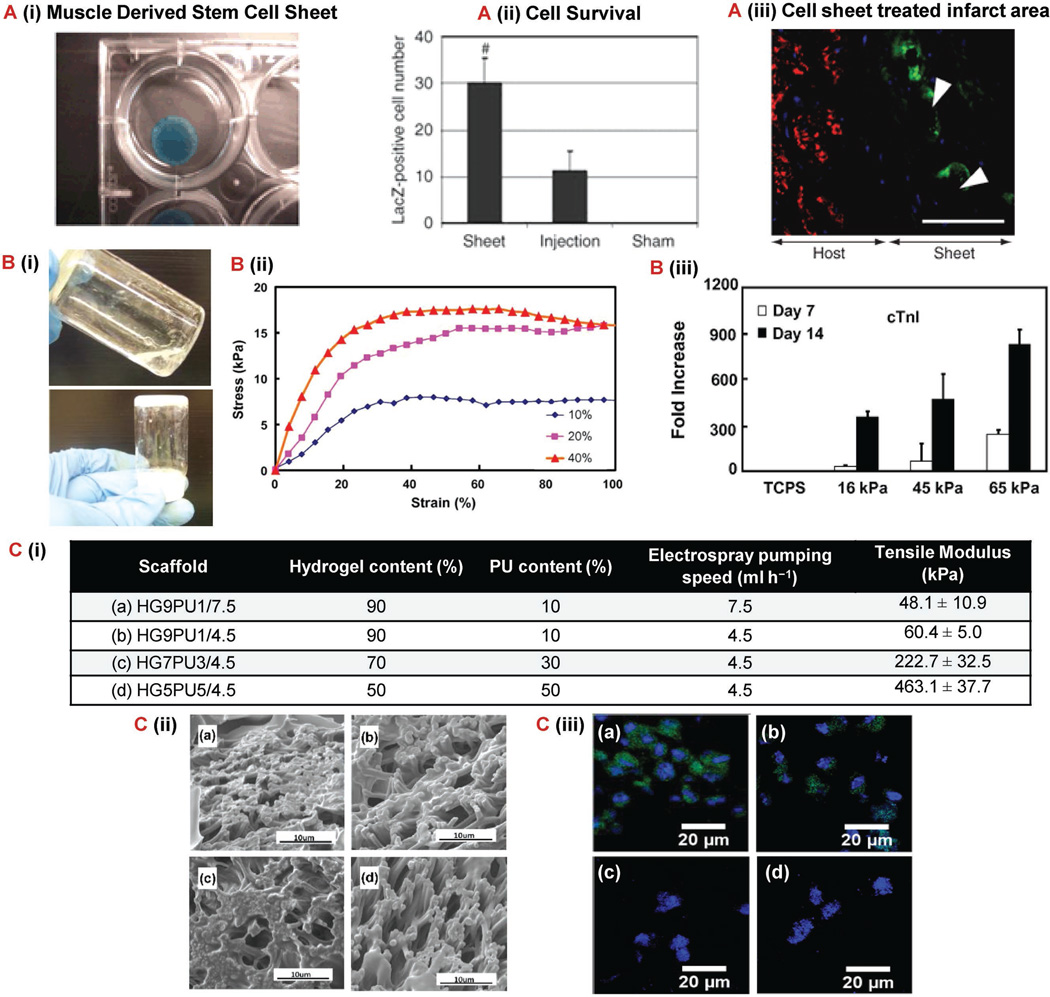
Alternatively, synthetic polymers are attractive because of the substantial control scientists have over tailoring their physical and chemical properties. Several hydrogels are currently being developed to mimic cardiac tissue properties and characteristics.[76] Elastomeric hydrogels, for example, have high elastic and tensile strength properties, which are needed in the tough myocardial environment.[77] Enhanced biomimetic properties are also seen in conductive hydrogels. These hydrogels have an improved ability to conduct electric signals and therefore, assist in maintaining a constant synchronization of the beating rates for grafted and native cardiac tissues. Scientist have also used separate synthetic polymers to create a composite scaffold using two different techniques in order to better mimic the native extracellular matrix.[78] Specifically, Xu et al. used electrospun polyurethane fibers in combination with a hydrogel based on N-isopropylacrylamide (NIPAAM), acrylic acid (AAc), 2-hydroxyethyl methacrylate (HEMA), and oligo (β-butyrolactone). They modulated several composition characteristics including global moduli, single-fiber moduli, fiber alignment and density. The bioactivity of these scaffolds was tested by electrospraying cardiosphere-derived cells and monitoring their differentiation toward cardiomyocytes. The results showed that although the cells attached to each scaffold composition relatively equally, the RT-PCR cardiac differentiation was enhanced with the lower scaffold modulus and highest fiber diameter as seen in Figure 1C. This study further confirms that finding the optimal composition and mechanical properties of scaffolds is important in order for stem cell therapy to reach therapeutic efficacy. Despite the fact that the mechanical properties of synthetic hydrogels can be relatively easy to tailor, their lack of bioactivity has proven to be a significant problem that scientists are continuing to work toward overcoming.
Figure 1.
Biomaterial-based approaches for improved cardiac stem cell therapy. A) MDSC sheet formed using temperature-responsive polymer (poly(N-isopropylacrylamide).[84] (i) Cell sheet containing MDSCs transduced with LacZ. (ii) Quantification of live LacZ-positive 12 weeks after implantation. (*p < 0.05 vs control, #p < 0.05 vs injection). (iii) Immunostaining of infarcted area for fsMHC-positive (green) in close proximity to Lac-Z-transduced MDSCs (white arrows) 12 weeks after implantation of MDSC sheet to show skeletal muscle-like formation. Scale bar: 50 µm. B) N-isopropylacrylamide, N-acryloxysuccinimide, acrylic acid, and poly (trimethylene carbonate)-hydroxyethyl methacrylate injectable thermosensitive hydrogel synthesized to improve MSC differentiation to cardiac cells.[89] (i) Image of hydrogel solution at 4 °C (top) and solid gel at 37 °C (bottom). (ii) Stress–strain curve for hydrogels with different concentrations of the three polymers. (iii) Cardiac gene cnT1 expression for MSCs affected by hydrogel moduli on hydrogels with varying concentration of the three polymers. (10% = 16 kPa, 20% = 45 kPa, and 40% = 65 kPa) (p < 0.05). C) Effect of NIPAAM/AAc/HEMA-oHB6 hydrogel and electrospun polyurethane fiber scaffold on cardiosphere-derived cell differentiation.[78] (i) Scaffold parameters for each of the four test groups. (ii) SEM image of each scaffold. (ii) cTnI expression (green) of CDCs in tissue constructs after 7 d of culture. Cell nuclei were stained by Hoechst (blue). Abbreviations: fsMHC, fast-skeletal myosin heavy chain; cnT1, cardiac troponin I; MDSC, muscle-derived stem cell; MSC, mesenchymal stem cell; VEGF, vascular endothelial growth factor; fsMDSC, fast skeletal myosin heavy chain; SEM, scanning electron microscopy.
4.1. Cell Sheets
The “Cell Sheet Engineering” approach to tissue engineering utilizes stacks of many individually harvested thin tissue layers to fabricate an integrated functional tissue in vitro.[79] In its early stages, this approach presented a “backward” technique due to the enzymatic harvesting steps that destroyed the engineered cells.[80] However, recent advances in scaffold coating strategies have allowed the easy detachment of cultured tissues from their “smart” scaffolds, keeping any developed cell-surface proteins and extracellular matrix connections intact.[80] The resulting stacked cell sheets resemble dense native cardiac tissue, and can be implanted without sutures through open heart surgery resulting in long-term survival and growth rate.[79,81] As a result, the cell sheet approach has become one of the most promising techniques in cardiac tissue engineering.[82] In fact, transplants of stacked cell sheets prepared from skeletal myoblasts into infarcted myocardium in MI rat models have shown positive results concerning vascularization and proliferation of healthy cardiac cells in the damaged areas.[83] Cell sheets derived from muscle stem cells have also been shown to yield better functional recovery in chronic infarcted myocardium than cell injections have.[84] As shown in Figure 1A, the cell sheets had a significant increase in cell survival, which then translated into a better therapeutic outcome in the form of reduced scar fractional area and increased formation of fast-skeletal myosin heavy chain positive myofibers. Bursac et al. discusses the high potential of cell sheets seeded with adipose derived MSCs, which after implantation into infarcted myocardium in rat models increases the myocardium wall thickness.[83]
Scientists use thermoresponsive coatings for the growing scaffold, which results in the successful growth of cardiac tissue in vitro for several reasons. First, each cardiomyocyte sheet consists of electrically and chemically coupled cells and can therefore maintain a synchronized pulsation.[81] In addition, although cell sheets initially lose pulsation after being stacked, synchronized beating is eventually restored and can be seen in macroscopic view.[81] Finally, the engineered graft area increases with host size after implantation, accompanied by an increase in conduction velocity and force. This is seen in implants of stacked myocardial layers in rats over a period of 24 weeks.[79] The most common thermoresponsive material used in the tissue engineering of cell sheets is poly(N-isopropylacrylamide) (PNIPAAm), which has a low critical solution temperature (LCST) of 32 °C.[80] This implies that the polymer is hydrophobic at temperatures above 32 °C, which encourages cell adhesion, and hydrophilic at temperatures below 32 °C, which encourages cell detachment.[79] These characteristics make the polymer ideal for tissue engineering applications; cells can be cultured on the PNIPAAm polymer at their nominal growth temperature of 37 °C and then safely harvested at a temperature below the LCST without damaging the cell-to-cell junctions or the extracellular matrix found between the harvested cells.[82] Another recent study showed that ASC cell survival in an ischemic heart can be increased by using thermoresponsive cell sheets.[85] These cell sheets were shown to improve ejection fraction as well as develop a new vascular network. Another example is the use of human amniotic fluid stem cells which have shown positive results when seeded on collagen hydrogels coated with temperature responsive material, such as methylcellulose hydrogels.[86] Transplantation of the obtained cell sheets increased the left ventricle size in infarcted rat models within four weeks.[86] Improved engraftment was also seen due to the increased extracellular matrix obtained from the cell sheet method.[86]
4.2. Injectable Hydrogels
Hydrogels are 3D networks of polymers that share several characteristics with natural extra-cellular environments.[77] They are hydrophilic and swell when exposed to water, and they can also promote the growth and adhesion of cardiovascular cells. Tissue engineering of cardiac tissue based on injectable hydrogels begins with encapsulating the cells.[87] This is achieved by mixing the cells and the matrix solution before incubating the solution for gelation.[81,87] The resulting gel is a homogeneous mixture consisting of cells, extracellular matrix proteins, and polymer. The gel is directly injected into the desired host cardiac tissue as a liquid and then polymerizes in the host tissue, becoming fixed.[81] The complete biodegradation of all polymer residues has been reported within six weeks after injection. Hydrogels have been reported to enhance stem cell retention and survival upon their injection into animal heart models.[88] Also, hydrogels provide an environment for cells where scientists can control the mechanical properties and, therefore, enhance stem cell differentiation. Li et al. encapsulated MSCs in a thermosensitive hydrogel made of N-isopropylacrylamide, N-acryloxysuccinimide, acrylic acid and poly(trimethylene carbonate)-hydroxyethyl methacrylate.[89] These hydrogels had varying moduli due to varying concentrations of the polymers used. The results showed that the cells encapsulated in the hydrogel with the highest modulus had a greater increase in MSCs differentiation to cardiomyocytes as seen in Figure 1B. Hydrogels that have been injected with hESC vascular cells show improved vascularization of infarcted heart areas in rat models and maintain myocardium contractile rates.[90] This has also been correlated with a decrease in area of the damaged site, viewed using magnetic resonance imaging techniques.[90] Due to their relative ease of delivery and tunable properties, injectable hydrogels hold a great promise for enhancing the efficacy of stem cell therapy to the heart.
4.3. Porous Scaffolds
Porous scaffolds are distinguished from hydrogels by their interconnected pores that allow the seeded cells to travel, proliferate, communicate, and form extracellular matrix components throughout the entire scaffold.[91] These pores also permit diffusion of nutrients and metabolic wastes. Pores are essential to cardiac tissue scaffolds as they increase vascularization, especially when combined with medium perfusion techniques that can enhance cardiac cell motion through the scaffold.[92] Scaffolds can be made porous through various techniques, depending on the material being used and the pore characteristics.[93] These techniques include freeze drying,[94] leaching,[95] 3D printing,[96] electrospinning,[97] and fiber extrusion.[98]
Scaffolds from natural and synthetic materials can be used in cardiac tissue engineering, although some have been shown to be more receptive to cardiac cell seeding than others.[99] According to Herrmann et al., polyurethane is identified as being among the best in the context of myocardial tissue engineering.[99] Fromstein et al. revealed the effect of different scaffold architectures on the development of cardiac patches from cardiomyocytes derived from embryonic stem cells.[100] Polyurethane scaffolds having different “macro-architectures” were fabricated using electrospinning or thermally induced phase separation (TIPS) techniques. Although both fabrication methods resulted in patches with contracting cells, only the stem cells seeded on the fibrous meshes from the electrospinning technique developed into the typical elongated morphology seen in cardiomyocytes derived from ESCs. Rai et al. developed a biomimetic PGS hydrogel sequentially treated with alkaline hydrolysis and acid and reported homogenous immobilization of the fibronectin and laminin peptide sequence.[101] It was shown that the porous PGS scaffold is biocompatible with rat CPCs and human cardiac MSCs. These results hold promise for PGS to serve as biomaterials for carriers of cells into the heart for cardiac tissue engineering. Ravichandran et al. discusses several comparable mechanical properties between PGS, fibrinogen core, shell scaffolds and native myocardial tissues.[102] Similarly, Prabhakaran et al. studied the embryonic stem cell behavior on a poly(d,l-lactide-co-glycolide)/collagen (PLGA/Col) scaffold versus a pure PLGA scaffold.[103] They found that the PLGA scaffold exhibited much higher tensile strength properties. However, the PLGA/Col scaffold proved to better support the interaction and growth of ESC differentiated cardiomyocytes. This highlights the importance of combining natural and synthetic polymers in the production of porous scaffolds in order to obtain a scaffold that can facilitate cell adhesion and provides ideal mechanical properties for cardiac tissue engineering.[93]
4.4. Cell-Surface Engineering with Bioactive Molecules
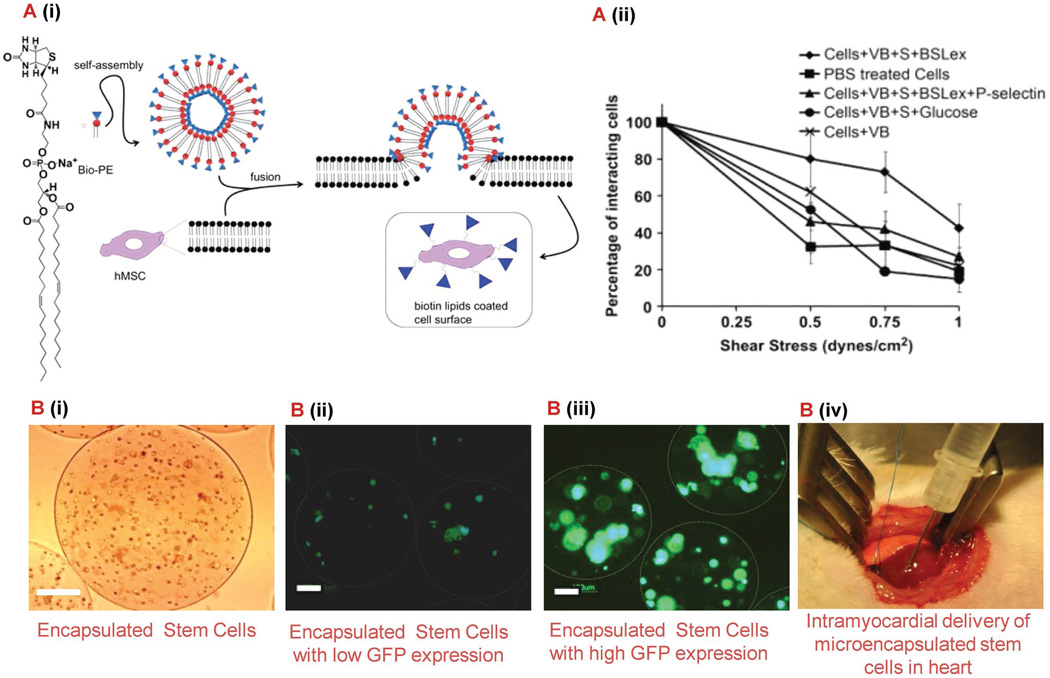
As mentioned previously, stem cells have the potential to regenerate tissue damaged by several cardiovascular diseases including myocardial infarction. However, to date, cell therapy has not reached the efficacy level needed and requires invasive surgeries to deliver cells to the target location. Ideally, a systemic infusion should be used, but only approximately 1% of the cells have been shown to home to the tissue of interest.[104] In addition, regardless of which delivery technique is used, scientists have observed that less than 10% of the injected stem cells are present in the heart 24 h following treatment.[105,106] Therefore, scientists have begun modifying the surface of stem cells in order to promote cell homing and engraftment at the treatment site. This can be accomplished by stimulating interactions between the stem cells and the endothelium typically used by hematopoietic stem cells and leukocytes that MSCs inherently lack.[104,107,108] These interactions lead to initial rolling and subsequent adhesion that results in transendothelial migration into the tissue. Specifically, Sarkar et al. used glycoprotein ligand-1 of the P-selectin active site called sialyl Lewis X (SLeX).[104] They attached a SLeX to MSCs using a biotin–streptavidin bridge after a lipid vesicle had been attached to the cell membrane using biontinylation. A schematic representation of the synthesis can be seen in Figure 2A. Their results showed that under shear stress the modified cells had a greater percentage of cells interacting with a P-selectin coated surface through both adhesion and rolling mechanisms. Although this method proved to be effective, streptavidin may cause an immune response and a method with less steps is more ideal. Cheng et al. used a simple procedure to chemically modified the MSC membrane using NHS-PEG2-maleimide as a membrane bound linker molecule and cysteine to provide the N-terminus thiol needed to react with maleimide.[107] After attaching a known strong binding peptide to the MSCs using this method it was shown that the cells targeted and adhered to E-selectin in an in vitro model of an inflamed blood vessel under physiological shear stress. In addition, this group proved that the binding kinetics of the peptide–selectin interaction can be modified in order to promote the MSCs to roll under physiological shear stress. To accomplish this they used a peptide that had a moderate binding IC50 with E-selectin to modify the stem cell membrane. This discovery is particularly important for the field because it validates theoretical models that had previously only been used to explain natural cell or bead rolling. This allows scientists in the future to refine theoretical models in order to develop better engineered cells for the purpose of targeting damaged tissue like damaged myocardium. It is important to note that both studies confirmed interactions were observed while the MSCs maintained their viability, proliferation, and differentiation ability. These studies prove that by modifying the surface of stem cells in accordance with specific ligands expressed by the target cell type, scientists have the ability to promote interactions leading to stem cell homing after systemic injection and increased retention regardless of which delivery route is chosen. Targeting the specified adhesion molecules mentioned above holds great promise for tissue regeneration due to the fact that both E and P-selections are overexpressed by inflammatory cytokine-activated endothelial cells present in damaged tissue including damaged myocardium. Recently, Chi et al. reported a pilot study in which they tested this theory using a porcine ischemia-reperfusion model.[105] Results showed that by incorporating a P-selectin glycoprotein ligand-1 mimetic onto the surface of cardiosphere-derived cells they were able to detect 28% of the cell population in the left anterior descending coronary artery region 3 h after injection. Although this study confirms the feasibility of using selectin-ligand functionalized cardiosphere-derived cells for targeted regeneration of damaged myocardium, future tests should be aimed at investigating the retention of the cells in the target region at longer time points in larger in vivo studies.
Figure 2.
Biomaterial-based delivery systems for enhanced cardiac stem cell therapy. A) Modified MSCs using biotinylated lipid vesicles for systemic cell targeting.[104] (i) Schematic of vesicle formation and MSC modification. (ii) Effect of shear stress on percentage of cell interactions with the P-selectin coated surface in a flow chamber assay at 0.5 dyn cm−2 . B) Microencapsulated stem cells for myocardial infarction therapy.[113] (i) hASCs encapsulated in alginate-based microcapsules. Scale bar: 50 µm. (ii, iii) Microencapsulated stem cells, genetically modified to express GFP (transduced with only baculovirus or PAMAM dendrimer coated baculovirus), under fluorescence microscope on day 2 post encapsulation. The white circles show the peripheral surface of the microcapsules. Scale bar: 100 µm. (iv) Photograph shows direct intramyocardial delivery of microencapsulated hASCs to the peri-infarct sites of rat heart. This strategy results in an improved cell retention, rapid angiogenesis, and improved cardiac function. Abbreviations: MSC, mesenchymel stem cell; hASCs, human adipose-derived stem cells; PAMAM, polyamidoamine; VEGF, vascular endothelial growth factor; PEG, polyethelene glycol; GFP, green fluorescent protein.
4.5. Polymeric Microcapsules
Although stem cell injections directly into the myocardium have been shown to promote positive remodeling of the tissue, the problem of cell retention still remains. Once injected, scientists have found that the contractile nature of the myocardium flushes the stem cells away from the site.[109] One method being studied in order to overcome these problems is the use of microcapsules as delivery systems for stem cells. As mentioned with the previous methods, a major benefit of using a polymeric delivery method such as microcapsules is that the mechanical and biological properties of the microcapsules can be manipulated based on polymer composition to influence stem cell behavior. However, due to the relatively small size of microcapsules, there are rarely concerns regarding adequate transport of oxygen and nutrient to the cells that other hydrogel techniques might encounter.[110] Several polymers have been used for such microcapsules, including extracellular matrix proteins,[111] PLGA,[112] alginate,[113] fibrinogen,[114] and chitosan[115] among several others. Specifically, Paul et al. recently used alginate microcapsules to encapsulate genetically modified human adipose-derived stem cells.[113] They used recombinant baculovirus and PAMAM dendrimers to transduce the cells with a gene to cause the overexpression of VEGF. As reported in Figure 2B, due to this overexpression of VEGF when the cells were injected into a rat model, significant positive remodeling was observed over a 10 week period in the form of increased injection fraction, fractional shortening, angiogenesis and arteriogenesis. Recently, Blocki et al. developed a composite microcapsule for the encapsulation and delivery of MSCs to the myocardium post myocardial infarction.[111] The biomaterials used for the microcapsule production were agarose, collagen I, fibrin, and dextran sulfate. The slow degradation profile of agarose allows for cells to be released into the damaged tissue in a controlled manner while the extracellular matrix proteins and dextran sulfate were chosen due to their ability to positively impact cell behavior. More specifically, the presence of dextran sulfate caused extensive cell spreading, proliferation and survival within the microcapsules. Following their material optimization, in vivo tests were carried out in a rat model where the microcapsules containing MSCs labeled with TAT peptide derivatized ultrasmall superparamagnetic iron oxide nanoparticles were injected into the peri-infarct and infarct region of the heart. A magnetic resonance signal indicating the presence of labeled MSCs could be detected up to 6 weeks in the groups that received the microencapsulated MSCs. However, at most, a weak signal was detected early in the group that received a single cell suspension. These results indicate that the microencapsulation of the MSCs did increase the cell retention in the damaged myocardium. Future studies assessing cardiac function post treatment would be beneficial in order to fully assess the therapeutic benefit of this treatment. Instead of using some of the natural polymers mentioned above, Lee et al. used PLGA in combination with polyethylenimine (PEI1.8k) in order to encapsulate MSCs in porous microcapsules.[112] PLGA was chosen due to its ideal degradation profile and PEI1.8k was added in order to enhance cellular retention due to electrostatic interactions. In vivo results showed that after injection into the damaged myocardium all three micro-encapsulated cell groups had a greater engraftment rate 2 weeks after treatment. However, as stated previously, further in vivo test should be performed in the future to assess how the cardiac function is affected by these positive results. In addition, in vivo testing over a longer time period, as seen in the previous examples, is needed to make an accurate assessment of the therapeutic potential of the treatment. These examples along with other studies provide evidence that microcapsules offer scientists with a viable method for enhancing stem cell therapy by providing a tunable delivery system.
5. Role of External Mechanical Stimuli on Cardiac Differentiation
Stem cells are unique in the fact that they differentiate based on the biological and mechanical cues they receive from their environment. Scientists have used this trait to their advantage by seeding cells on substrates with specific mechanical properties, and also by subjecting the cell seeded substrates to mechanical stimulation. For a tissue engineering approach, scientists use bioreactors as an attempt to improve the engineered cardiac tissue by driving it to mimic native cardiac muscle behavior.[116] Subjecting stem cells to biomimetic signals also improves cardiac gene expression in differentiated stem cells.
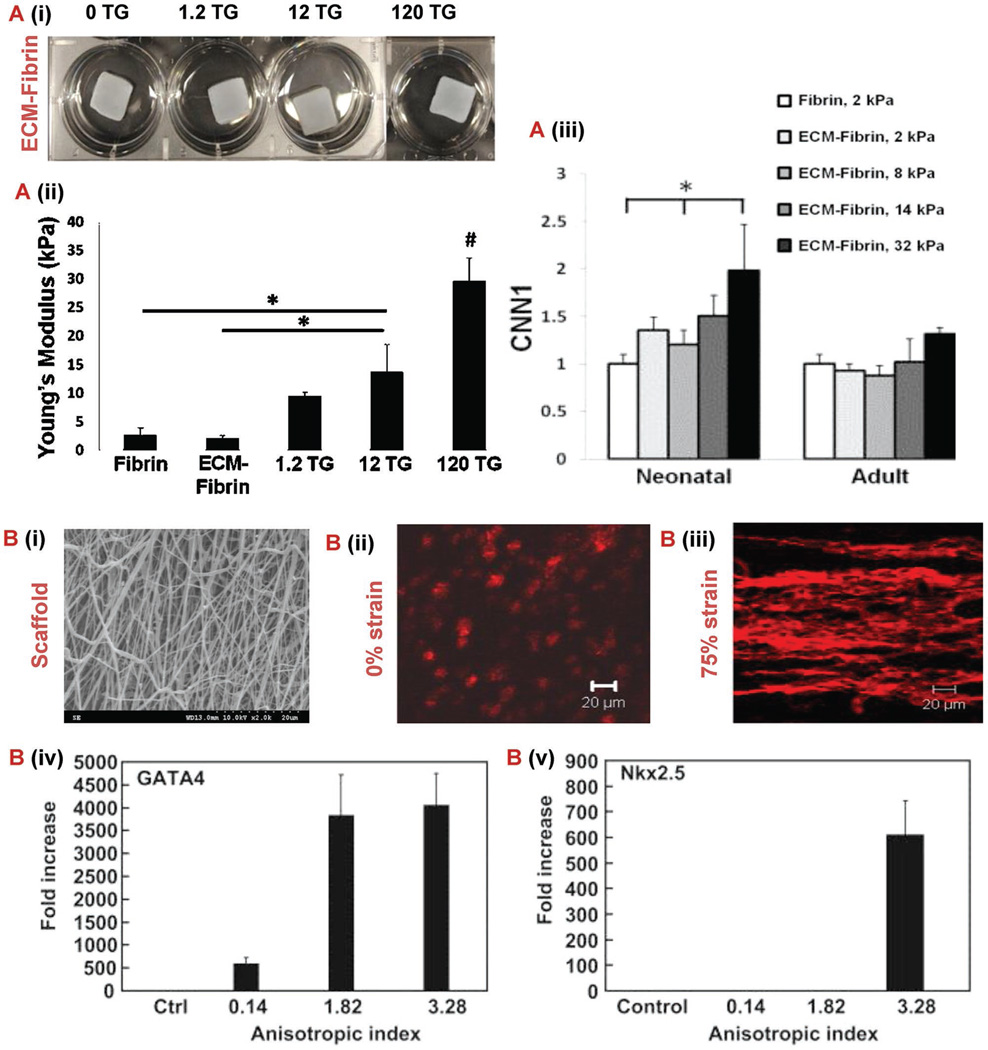
When designing a device for stem cell delivery, the fabrication method and material properties can greatly influence the stem cell fate.[117] This is called mechanotransduction, and is when the mechanical properties of the material evoke a physical stimulus that translates into a biological response in the cells. Scientists have used this to their advantage when choosing the materials to use for stem cell delivery devices. For example, Williams et al. studied how the modulus of hydrogels with different compositions of ECM and fibrin affected the behavior of C-kit+ progenitor cells.[118] As seen in Figure 3A, as the moduli of the substrate increased the cardiovascular gene expression increased as well. This indicates that stiffer substrates provoke this type of progenitor cell to differentiate toward a cardiac lineage. Another example of stem cell fate dictated by substrate properties can be seen in the study by Sreerekha et al., where scientists observed how the diameter of electrospun PLGA and PLGA-fibrin fibers influenced MSC behavior.[119] This study proved that a composite scaffold with fibrin nanofibers ranging from 50 to 300 nm and PLGA microfibers ranging from 2 to 4 µm enhanced MSC differentiation into cardiomyocytes and proliferation. This provides evidence that a substrate with a heterogeneous network of fibers provides a more ideal environment for cardiomyocytes to thrive.
Figure 3.
Mechanobiology to control stem cell. A) Characterization of ECM-fibrin gels with varying concentrations of TG.[101] (i) Young’s modulus of gels produced by uniaxial testing. (ii) Swelling ratio. (iii) Cardiovascular gene expression of smooth muscle marker CNN1 and endothelial in gels with either neonatal ECM or adult ECM 21 d after cardiac progenitor cell seeding. B) (i) Anistroic fiber morphology shown in 2000× SEM micrograph of poly(ester carbonate urethane) electrospun scaffold.[123] (ii, iii) Z-stack confocal images of tissue constructs at different strains (0% and 75%). Rhodamine phalloidin was used to stain F-actins of the cells. Scale bar: 20 µm (iv, v) Real time RT-PCR analysis of cardiac specific genes GATA4 and Nkx 2.5. Control group represents the gene expression of MSCs culture on tissue culture plates. Control group values were used for normalization. Abbreviations: SEM, scanning electron microscopy; RT-PCR, reverse transcription polymerase chain reaction; TG, transglutaminase; ECM, extracellular matrix; CNN1, calponin 1.
Another method for influencing stem cell fate is subjecting the cells to mechanical stimulation using a bioreactor. The first type of bioreactor was used to mimic the physical and mechanical stimulations found in the environment of native cardiac muscles.[120] This conditioning increases the force of contraction generated in rat cardiac constructs revealing the importance of mechanical bioreactors in engineering biomimetic cardiac muscles.[116,121] Mechanical simulation of engineered cardiac tissues has also been shown to enhance cardiomyocyte proliferation.[121] In fact, elastic properties have been improved by seeding mouse embryonic stem cells onto poly(lactide-co-caprolactone) scaffolds and stimulating the scaffold using cyclic stretch bioreactors.[90] Guo et al. demonstrated that stretching cardiac patches fabricated from differentiated ESCs also results in synchronous beating and response to environmental changes.[122] Proper stimulation of stem cells is a vital step in the development of cardiac patches because their differentiation into a certain cell line is determined by the signals they are subjected to.[83] For example, imposing a 2 Hz cyclic stretch over a period of 7 d was shown to prevent tumor development in cardiac patches developed from embryonic stem cells.[83] Another example was shown by observing the effect of static strain on a MSC seeded electrospun poly(ester carbonate urethane) scaffold.[123] The electrospinning technique allowed scientists to fabricate a scaffold that mimicked the anisotropic and biaxial mechanical properties of native porcine myocardium. As shown in Figure 3B, the magnitude of static strain greatly affected both the cell morphology and differentiation. The anisotropic index of the tissue construct without static stain was 0.14. However, after applying a 75% static strain the anisotropic index changed to 3.28 and the difference in cellular alignment can clearly be visualized in the Figure. Even more intriguing is the dramatic upregulation of key early cardiac differentiation genes GATA4 and Nkx2.5 that was detected. When compared to the MSCs cultured on standard tissue plates, the 75% strained tissue constructs exhibited approximately a 4000 and 600 fold increase of GATA4 and Nkx2.5 expression, respectively. These results clearly demonstrate the immense potential of using mechanical stimulation to engineer a tissue construct that mimics both the mechanical and cellular properties of the myocardium. Current bioreactors are sophisticated, large, unreliable, and difficult to operate and maintain.[121] This prevents their use in the mass-production of cardiac patches and limits them to experimental applications. Nevertheless, recent advances in bioreactor technologies have allowed the recreation of the native cardiac environment in the lab by providing the needed mechanical, biochemical and electrical signals.[9] In addition, the emergence of several prototypes for portable micro-bioreactors[124] with minimal pumps, valves, and accessories[125] improves the potential of tissue engineering to eventually meet the clinical need and mass production of cardiac patches.[126] Biochemical stimulation can also be used. Bioreactors within this category are denoted by “spatial control” reactors, since they are used to deliver growth factors and molecules in specific amounts within the required cells only.[127] Biochemical stimulation enhances the vascularization of cardiac patches engineered from rat models, which increases the amount of oxygen supplied to the cardiomyocytes to the required level found in native tissues.[116]
Lastly, cardiac patches are subjected to electrical bioreactors. Electrical stimulation of engineered cardiac tissue aligns cardiomyocytes seeded onto scaffolds.[121] In addition, it enhances the electrical conductivity properties of the engineered cardiac muscle by increasing the electrical coupling between the cells, which increases the conduction velocity and establishes a rate comparable to that of native cardiac tissues.[121] This has been shown through several experiments performed on rat and mouse cardiac patch models which highlights the ability to engineer “pacemaker” cardiomyocytes.[9,121]
Cyclic stretching, stretching, Percoll gradient centrifugation, and electric field stimulation are some of the common bioreactors and stimulation techniques used in preparing cardiac patches from various stem cell sources.[83,122] These techniques can also be helpful in stem cell proliferation and expansion in vitro.[122] Some of the most important types of bioreactors are outlined in Table 2, along with their potential applications and benefits. Although there are significant obstacles associated with the use of bioreactors as mentioned above, this technology provides a valuable tool for scientists to engineer more sophisticated cardiac patches in vitro prior to implantation. This has the potential for these types of devices to have a reduced risk of failure when transplanted into the human body.
Table 2.
Different types of bioreactors for cardiac tissue engineering.
| Bioreactors | Culture type | Advantages | Examples | Ref. |
|---|---|---|---|---|
| Perfusion | Scaffolds | Enhances mass transfer of oxygen through the engineered patch |
Vascular cells reveal changes in their metabolic and functional properties after exposure to increased levels of shear stress, as well as increased cell distribution and lower diffusion gradients |
[120,143] |
| Creates frictional shear stress on the cells which enhances cell proliferation |
||||
|
Multi-shear perfusion |
Scaffolds | Delivers different shear stresses at the same time |
DNA content of cultured cells increases by 91% after exposure to the bioreactor |
[120] |
| Can be used on 3D tissues | ||||
| Pulsatile fow | Cell-Sheets | Enhances vascularization of stacked cell sheets |
Stacking of six layers of rat cardiac cell sheets resulted in a thicker and denser grafts, which contracts regularly after implantation into host rat |
[120] |
|
Uniaxial cyclic stress |
Scaffolds | Increases cardiomyocyte size | Human cardiac constructs implanted into rats after exposure to uniaxial loading displayed increased active force and enhanced graft perfusion into host tissue |
[120] |
| Aligns fibers in the ECM | ||||
| Increases angiogenesis | ||||
|
Rotational wall vessel |
Cell-culture vessels |
Creates laminar fluid flow | Cardiomyocytes cultured in these bioreactors have a constant pH, %CO2, and %O2, compared to an increasing amount of DNA in the culture. |
[143] |
| Enhances mass transfer rate | ||||
|
Electrical stimulation |
Scaffolds and hydrogels |
Ensures impulse propagation | Cardiac constructs propagate continuous pulses and a rate of 400 beats min−1 after inserting in an electrical stimulation bioreactor |
[144] |
| Increased conduction velocities to mimic in vitro conditions |
||||
| Caused synchronous macroscopic pulses |
6. Implementing Microscale Techniques for Cardiac Tissue Engineering
Cardiac cell engraftment and retention has remained variably low after transplantation of tissue constructs into the heart. One main reason is the insufficient flow of oxygen and nutrients that is required through an intricate micro-vessel network. The formation of this network is currently slow, resulting in dying cells because of the lack of blood vessels in the proximity. Microfluidics offers some remedies for this problem in the area of tissue engineering by enabling scientists to control the spatial distribution of cells and the chemical gradients. Scientists can also induce flow signaling and transduction, thereby achieving high precision in the reproduction of the cardiac in vivo microenvironment.[128] This section will focus on different methods in which scientists are creating vascular networks using microfluidics for cardiac tissue engineering.
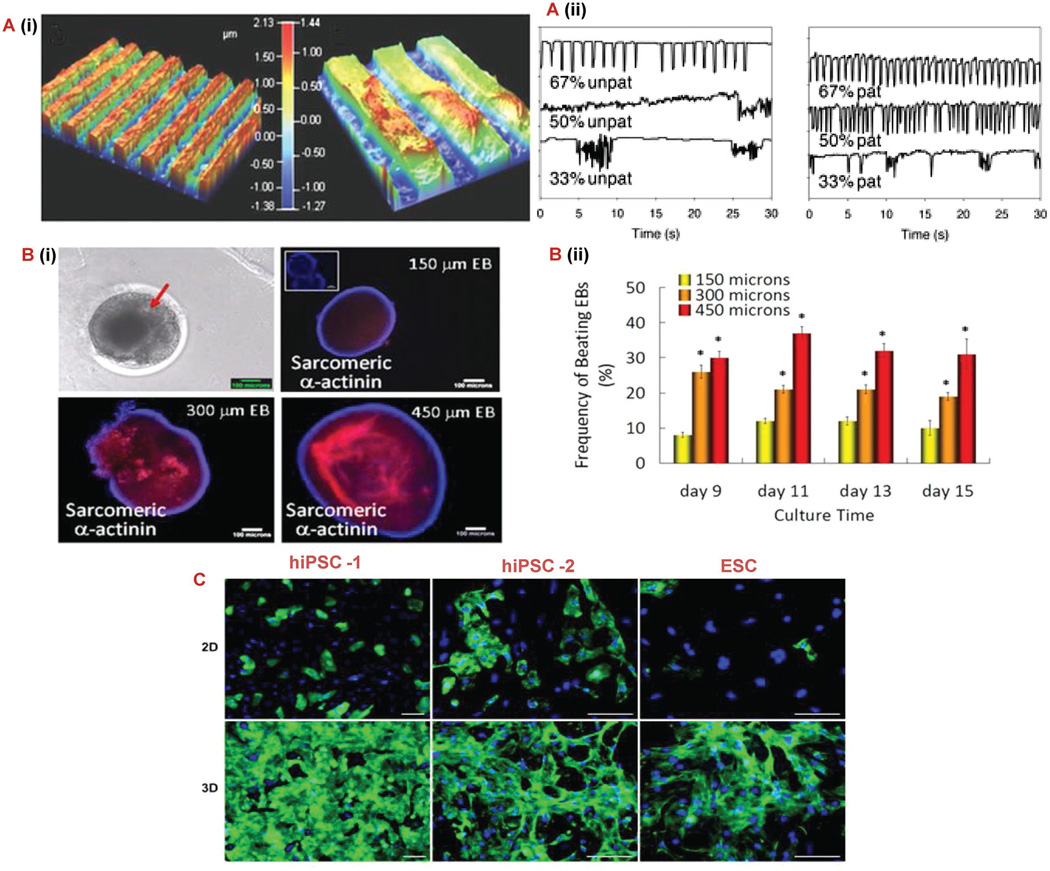
In cardiac tissue engineering, vascularization is a crucial element for cell survival and improvement of cardiac function. Prevascularization and angiogenesis/vasculogenesis are two methods that are used to construct vessel networks. Prevascularization aims at constructing branched perfusable blood vessel networks in the cardiac cell tissue prior to implantation. The vessel network is designed such that anastomosis occurs instantly upon implantation. With this prevascularization technique researchers have gained a precise control over the spatial arrangement of the vessel network. Microfluidics offers a number of techniques for the in vitro formation of vessel networks which can be grouped under (1) subtractive methods (needle-based molding method or dissolvable network-based sacrificial molding[129]), (2) additive methods (bonding of preformed hydrogel slabs) and (3) hybrid methods (bioprinting).[130] Continuous delivery of oxygen and nutrients, continuous removal of metabolic wastes, high rate of vascular anastomosis, immediate perfusion, and maturation upon implantation are major advantages of prevascularization. In vasculogenesis and angiogenesis, the scaffold is prepared in vitro using techniques that enhance blood vessel formation in vivo. Preparation techniques for vessel formation using microfluidics technologies include (1) micropatterning, (2) use of functionalized biomaterials, (3) induction of gradient of growth factors and (4) control of cell to cell interactions using co-culture of multiple cell types.[129,130] Micropatterning can also be used to guide the proliferation and alignment of stem cells in order to create a more functional tissue.[131] For example, Giridharan et al. cultured embryonic cardiomyoblasts on a thin poly-dimethyl-siloxane (PDMS) synthetic polymer membrane in a device modeling the left ventricle resulting in a construct that succeeded in simulating diastolic loading.[132] Tsang et al. proved this concept by using methacrylated gelatin to create a micropatterned hydrogel and subsequently seeded the construct with cardiomyocytes.[131] As seen in Figure 4A, this micropatterning produced cells with more regular beating characteristics, which is a quality that cardiomyocytes need to up hold in order for the heart to function properly. Along with micropatterning, microwells have also been shown to control the fate of stem cells due to the cells growing a spheroid bodies instead of in sheets. Recently, Yu-Shik et al. discovered that the size of the wells and, therefore, the size of the cell body grown influence which cell lineage stem cells differentiate into.[133] They used embryonic bodies for their study and found that the larger the cell bodies exhibited cardiogenesis (Figure 4B) and the smaller cell bodies differentiated to an endothelial cell lineage. Another study compared the maturity of cardiomyocytes differentiated from human pluripotent stem cells (hPSCs) cultures in 2D sheets and 3D cardio-spheres.[134] As seen in the images of Figure 4C, the hPSCs that were grown as aggregates showed a significant increase in cardiomyocyte characteristics. Specifically, the cell aggregates produced more homogenous population of cardiomyocytes with better intracellular calcium transients and contractility. These studies provide further evidence that stem cell differentiation can be influenced by several environmental factors that scientists can use to their benefit for specific regenerative purposes.
Figure 4.
Microscale techniques for controlling stem cell behavior. A) (i) Optical profilometry of patterned 67% PD gelatin hydrogel. A) (ii) Cardiomyocyte beating characteristic of unpatterned and patterned gelatin gels at varying PD concentrations. Pixel intensity was used to monitor the gels over a 30 s period [131] B) (i, ii) Morphology of beating EBs, immunocytochemical characterization of cardiomyogenic differentiation identified by sarcomeric α-actinin and evaluation of beating EBs cultured in microwells. Inset for 150 µm EB figure indicates control stained only with secondary antibody. Scale bar: 100 µm.[133] C) Immunostaining for sarcomeric α-actinin (green) and nuclei (blue) of three different pluripotent cell sources harvested from day 21 monolayer cultures and day 7 cardiosphere.[138] Scale bars: 50 µm. Abbreviations: hiPSC, human induced pluripotent stem cell; hESCs, human embryonic stem cell; PD, photodegradable; EB, embryonic bodies.
Biomaterials strongly determine the limitations of these technologies.[145] Starting with silicon etching, microfluidic cardiac tissue engineering later evolved to use PDMS scaffolds, which ensure high fidelity and high feature resolution but offer only low cell attachment. Annabi et al. devised a method to coat the PDMS channel walls with tropoelastin-based hydrogel layer and observed an increase in cell attachment.[135] Increase in cell attachment levels led them to anticipate that the method may be useful to produce elastic tissues such as blood vessels.[135] However, since PDMS is not biodegradable, some applications moved to poly(lactide coglycolide) PLG and polyglycerol sebacate (PGS).[129] PGS is believed by some to be superior in properties to PLG, because the latter swells in vivo and causes chronic inflammation while PGS degrades slowly mainly due to surface erosion.[136] An application involving PGS with a built-in vascular network showed the successful biodegradation of PGS with an infiltration of host cells into the vessel network but without any immediate anastomosis upon implantation.[137] Hydrogels were also introduced into these applications, which provided several advantages since they allow large biomolecule and gas exchange, remove the 3D constraints on cell interaction,[129] as well as, facilitate the control over the physical, mechanical and biological microenvironments.[130]
The microfluidic and micropatterning techniques mentioned in this section offer a valuable strategy to solve the common problem in tissue engineering of inadequate vascularization.[146,147] The spatial control over biomaterials, cells, and biomolecules provides the opportunity for scientists to engineer a tissue construct with a vasculature network more indicative of native myocardium prior to implantation. This increases the chances for success in the treatment by reducing cell death due to lack of proper oxygen, waste, and nutrient transport. Hydrogels are the most common biomaterials used today for microfluidic applications in cardiac tissue engineering, however, challenges still remain in finding the biomaterial that is superior in all the characteristics needed for microfluidic devices. Furthermore, although this technology has made great strides, concerns still remain in inadequate engraftment of cardiac patches after transplantation. Lastly, in order for cardiac patches to become clinically viable the thickness of the patches must increase while still maintaining the mature prevascularization discussed in this section.
7. Conclusion and Future Outlook
Research for cardiac tissue engineering and regenerative medicine with stem cells is greatly advancing with the use of biocompatible materials further increasing their therapeutic efficacy. Accessible sources of stem cells for cardiac applications have been located but their survival in vivo after specialization has been continuously reported to be modest. Several types of stem cells have been identified: embryonic stem cells which are subject to ethical issues, adult stem cells which are difficult to locate, extract and separate from their heterogeneous environment and iPSCs which are easily accessible, expandable, patient specific and have so far proven to have therapeutic potential. Moreover, the challenge of translating these cell types into a clinical setting in a timely and cost effective manner still remains. Studies have shown that combining multiple cell types with growth factors in cardiac therapies generates better results, however, there still has not been a specific combination that has had adequate regenerative capacity for clinical translation. In addition, recently the hypothesis that paracrine factors are the primary mediators of cardiac regeneration by stem cells has gained support among scientists. However, there are still many unknown paracrine and differentiation mechanisms regarding stem cell behavior in the cardiac microenvironment. Future studies dedicated to understanding these mechanisms would provide valuable insight into treatment parameters and designs. Choosing the optimal route is crucial for a successful treatment, and is done based on the specific clinical case. Among the available routes for stem cell delivery (intracoronary, intravenous, intramyocardial, retrograde coronary sinus and intrapericardial), the predominant delivery methods in clinical trials today are intracoronary and intramural route of administration. In tissue engineering, the different methodologies that combine cells and materials (cell sheets, porous scaffolds, injectable hydrogels, cell surface engineering, and microcapsules) have each succeeded in enhancing cardiac repair. Nevertheless, in order to make these treatments easier to translate into the clinical setting the development of minimally invasive delivery techniques will be important. The main issues remain with improving the electrical, chemical and biomechanical properties of the cardiac construct to match those of the heart as well as increase the functionality and speed of formation of the vessel network within the construct. Bioreactors, which are designed to test the prepared cell cultures by simulating the in vivo environment, allow for improvement of the construct designs within labs. However, they are still too sophisticated to enter mass production. Future studies are turned toward understanding the behavior of the cultured stem cells in 3D constructs and controlling their environment through time and space. Recent advances in cardiac repair are directed at designing patient-specific supports for clinical applications. While no generally accepted solution for stem cell based cardiac therapy has been reached yet, the field of research is advancing quickly with more promising technology and results.
Acknowledgments
Anwarul Hasan acknowledges the startup grant and the University Research Board (URB) grant from American University of Beirut, Lebanon, and the National Council for Scientific Research (CNRS) grant, Lebanon, as well as the Farouk Jabre interdisciplinary research award. Arghya Paul acknowledges the University of Kansas New Faculty General Research Fund for support and assistance with this work. The authors also acknowledge an investigator grant provided by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the NIH Award Number P20GM103638-04 (to A.P.). R.W. acknowledges the financial support from NIGMS (NIH, T32-GM008359) Biotechnology Predoctoral Research Training Program.
Contributor Information
Prof Anwarul Hasan, Email: ahasan@qu.edu.qa, Department of Mechanical and Industrial Engineering, College of Engineering, Qatar University, Doha, Qatar; Biomedical Engineering and Department of Mechanical Engineering, Faculty of Engineering and Architecture, American University of Beirut, Beirut 1107 2020, Lebanon; Center for Biomedical Engineering, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA 02139, USA; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Prof Renae Waters, BioIntel Research Laboratory, Department of Chemical and Petroleum Engineering, Bioengineering Graduate Program, School of Engineering, University of Kansas, Lawrence, KS, USA.
Prof Boustany Roula, Biomedical Engineering and Department of Mechanical Engineering, Faculty of Engineering and Architecture, American University of Beirut, Beirut 1107 2020, Lebanon.
Prof Rahbani Dana, Biomedical Engineering and Department of Mechanical Engineering, Faculty of Engineering and Architecture, American University of Beirut, Beirut 1107 2020, Lebanon.
Prof Seif Yara, Biomedical Engineering and Department of Mechanical Engineering, Faculty of Engineering and Architecture, American University of Beirut, Beirut 1107 2020, Lebanon.
Prof Toubia Alexandre, Biomedical Engineering and Department of Mechanical Engineering, Faculty of Engineering and Architecture, American University of Beirut, Beirut 1107 2020, Lebanon.
Prof Arghya Paul, Email: arghyapaul@ku.edu, BioIntel Research Laboratory, Department of Chemical and Petroleum Engineering, Bioengineering Graduate Program, School of Engineering, University of Kansas, Lawrence, KS, USA.
References
- 1.Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, Sayegh M, Hossain MM, Paul A. Adv. Sci. 2015;2 doi: 10.1002/advs.201500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matar AA, Chong JJ. SpringerPlus. 2014;3:440. doi: 10.1186/2193-1801-3-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jukes J, Both S, Post J, Blitterswijk Cv, Karperien M, Boer Jd. In: Tissue Engineering. Blitterswijk CV, Thomsen P, Lindahl A, Hubbell J, Williams DF, Cancedda R, Bruijn JDd, Sohier J, editors. Burlington: Academic Press; 2008. p. 1. [Google Scholar]
- 4.Cimetta E, Vunjak-Novakovic G. Exp. Biol. Med. 2014;239:1255. doi: 10.1177/1535370214530369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Burridge PW, Kropp EM, Chuppa SL, Kwok WM, Wu JC, Boheler KR, Gundry RL. J. Visual. Exp. 2014 doi: 10.3791/52010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim PJ, Mahmoudi M, Ge X, Matsuura Y, Toma I, Metzler S, Kooreman NG, Ramunas J, Holbrook C, McConnell MV. Circ. Res. 2015;116:e40. doi: 10.1161/CIRCRESAHA.116.304668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutts J, Nikkhah M, Brafman DA. Biomarker Insights. 2015;10:77. doi: 10.4137/BMI.S20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu W-Z, Hauch KD, Xu C, Laflamme MA. Transplant. Rev. 2009;23:53. doi: 10.1016/j.trre.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer RK, Chiu LLY, Reis LA, Radisic M. Curr. Opin. Biotechnol. 2011;22:706. doi: 10.1016/j.copbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal A, Gálvez B. Stem Cell Rev. Rep. 2013;9:814. doi: 10.1007/s12015-013-9461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S. Nat. Biotechnol. 2007;25:1015. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 12.Shi C, Li Q, Zhao Y, Chen W, Chen B, Xiao Z, Lin H, Nie L, Wang D, Dai J. Biomaterials. 2011;32:2508. doi: 10.1016/j.biomaterials.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Circulation. 2008;118:507. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Circ. Res. 2009;104:e30. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, Hofmann F, Seyfarth M, Sinnecker D, Schömig A, Laugwitz KL. N. Engl. J. Med. 2010;363:1397. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Zhou J, Wang H, Zhao M, Wang C. Regenerat. Med. Res. 2013;1:6. doi: 10.1186/2050-490X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdi J, Tan A, Shoae-Hassani A, Seifalian AM. J. Biol. Eng. 2014;8 doi: 10.1186/1754-1611-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Biochem. Biophys. Res. Commun. 2004 Feb 6;314:420. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 19.Russo V, Young S, Hamilton A, Amsden BG, Flynn LE. Biomaterials. 2014;35:3956. doi: 10.1016/j.biomaterials.2014.01.075. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda K. Artif. Org. 2001;25:187. doi: 10.1046/j.1525-1594.2001.025003187.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim SW, Zhang HZ, Kim CE, Kim JM, Kim MH. Int. J. Cardiol. 2013;168:1062. doi: 10.1016/j.ijcard.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Telukuntla KS, Suncion VY, Schulman IH, Hare JM. J. Am. Heart Assoc. 2013 Oct;10(2):e000338. doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Cell. 2003;114:763. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 24.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Proc. Natl. Acad. Sci. USA. 2003;100:12313. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Nature. 2005;433:647. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Circulation. 2007;115:896. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O’Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Basic Res. Cardiol. 2011;106:849. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. J. Am. Coll. Cardiol. 2005;46:1339. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 29.Yoder MC. Cold Spring Harb. Perspect. Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Nat. Med. 2001;7:430. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 31.Nian M, Lee P, Khaper N, Liu P. Circ. Res. 2004;94:1543. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 32.N. Engl. J. Med. 2004;351:209. [Google Scholar]
- 33.Bui Q, Gertz Z, Wilensky R. Stem Cell Res. Ther. 2010;1:29. doi: 10.1186/scrt29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui QT, Gertz ZM, Wilensky RL. Stem Cell Res. Ther. 2010;1:29. doi: 10.1186/scrt29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wollert K, Drexler H. Nat. Rev. Cardiol. 2010;7:204. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 36.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Basic Res. Cardiol. 2008;103:525. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 37.Sheng CC, Zhou L, Hao J. BioMed. Res. Int. 2013;2013:15. doi: 10.1155/2013/547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. Cells Tissues Organs. 2001;169:12. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 39.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH. Circulation. 2003;108:863. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 40.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. J. Nucl. Med. : Off. Publ. Soc. Nucl. Med. 2006;47:1295. [PubMed] [Google Scholar]
- 41.Dib N, Menasche P, Bartunek JJ, Zeiher AM, Terzic A, Chronos NA, Henry TD, Peters NS, Fernandez-Aviles F, Yacoub M, Sanborn TA, Demaria A, Schatz RA, Taylor DA, Fuchs S, Itescu S, Miller LW, Dinsmore JH, Dangas GD, Popma JJ, Hall JL, Holmes DR., Jr JACC Cardiovasc. Intervent. 2010;3:265. doi: 10.1016/j.jcin.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Campbell NG, Suzuki K. J. Cardiovasc. Transl. Res. 2012;5:713. doi: 10.1007/s12265-012-9378-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Q, Sun Y, Xia L, Chen A, Wang Z. Ann. Thorac. Surg. 2008;86:1833. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 44.Perin E, Silva G, Fernandes M, Munger T, Pandey A, Sehra R, Talcott M, Bichard C, Creed J, Wong J, Oliveira E, Zheng Y, Canales J, Cardoso C, Patterson M, Serruys P. EuroIntervention. 2007;3:142. [PubMed] [Google Scholar]
- 45.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P. Circulation. 2006;114:I108. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 46.Eke CC, Gundry SR, Fukushima N, Bailey LL. Am. Surg. 1997;63:417. [PubMed] [Google Scholar]
- 47.Menasche P, Kural S, Fauchet M, Lavergne A, Commin P, Bercot M, Touchot B, Georgiopoulos G, Piwnica A. Ann. Thorac. Surg. 1982;34:647. doi: 10.1016/s0003-4975(10)60904-6. [DOI] [PubMed] [Google Scholar]
- 48.Raake P, vonDegenfeld G, Hinkel R, Vachenauer R, Sandner T, Beller S, Andrees M, Kupatt C, Schuler G, Boekstegers P. J. Am. Coll. Cardiol. 2004;44:1124. doi: 10.1016/j.jacc.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 49.Jordan JE, Zhao ZQ, Vinten-Johansen J. Cardiovasc. Res. 1999;43:860. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. N. Engl. J. Med. 2000;342:626. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 51.Heba G, Krzeminski T, Porc M, Grzyb J, Dembinska-Kiec A. J. Physiol. Pharmacol. 2001;52:39. [PubMed] [Google Scholar]
- 52.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Lancet. 2003;362:697. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 53.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Circulation. 2004;110:3300. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 54.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Proc. Natl. Acad. Sci. USA. 2005;102:3766. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Circulation. 2003;107:1322. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 56.Penn MS. Circ. Res. 2009 May 22;104:1133. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS. Circulation. 2012;126:314. doi: 10.1161/CIRCULATIONAHA.111.082453. [DOI] [PubMed] [Google Scholar]
- 58.Formiga FR, Pelacho B, Garbayo E, Abizanda G, Gavira JJ, Simon-Yarza T, Mazo M, Tamayo E, Jauquicoa C, Ortizde-Solorzano C. J. Control. Rel. 2010;147:30. doi: 10.1016/j.jconrel.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 59.Formiga FR, Pelacho B, Garbayo E, Imbuluzqueta I, Díaz-Herráez P, Abizanda G, Gavira JJ, Simón-Yarza T, Albiasu E, Tamayo E, Prósper F, Blanco-Prieto MJ. J. Control. Rel. 2014;173:132. doi: 10.1016/j.jconrel.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 60.Tompkins J, Riggs A. Front. Biol. 2015;10:11. [Google Scholar]
- 61.Correia C, Serra M, Espinha N, Sousa M, Brito C, Burkert K, Zheng Y, Hescheler J, Carrondo MJT, Šarić T, Alves PM. Stem Cell Rev. 2014;10:786. doi: 10.1007/s12015-014-9533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bianco P, Robey PG. Nature. 2001;414:118. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 63.Bhuvanalakshmi G, Arfuso F, Dharmarajan A, Warrier S. Stem Cell Rev. Rep. 2014;10:856. doi: 10.1007/s12015-014-9530-3. [DOI] [PubMed] [Google Scholar]
- 64.Lu T-Y, Yang L. Stem Cell Res. Ther. 2011;2:44. doi: 10.1186/scrt85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reis LA, Chiu LL, Liang Y, Hyunh K, Momen A, Radisic M. Acta Biomater. 2012;8:1022. doi: 10.1016/j.actbio.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 66.Pok S, Myers JD, Madihally SV, Jacot JG. Acta biomaterialia. 2013;9:5630. doi: 10.1016/j.actbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng K, Blusztajn A, Shen D, Li T-S, Sun B, Galang G, Zarembinski TI, Prestwich GD, Marbán E, Smith RR. Biomaterials. 2012;33:5317. doi: 10.1016/j.biomaterials.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Godier-Furnémont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, Homma S, Vunjak-Novakovic G. Proc. Natl. Acad. Sci. 2011;108:7974. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Shi J, Wang Y, Yin Y, Wang L, Liu J, Liu Z, Duan C, Zhu P, Wang C. Biomaterials. 2014;35:3986. doi: 10.1016/j.biomaterials.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Panda NC, Zuckerman ST, Mesubi OO, Rosenbaum DS, Penn MS, Donahue JK, Alsberg E, Laurita KR. J. Int. Cardiac. Electrophysiol. 2014;41:117. doi: 10.1007/s10840-014-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tous E, Purcell B, Ifkovits JL, Burdick JA. J. Cardiovasc. Transl. Res. 2011;4:528. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 72.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Tissue Eng. 2005;11:1860. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 73.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Biomaterials. 2013;34:5813. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ravichandran R, Seitz V, Reddy Venugopal J, Sridhar R, Sundarrajan S, Mukherjee S, Wintermantel E, Ramakrishna S. Macromol. Biosci. 2013;13:366. doi: 10.1002/mabi.201200391. [DOI] [PubMed] [Google Scholar]
- 75.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Tissue Eng. Part B, Rev. 2010;16:169. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paul A. Nanomedicine. 2015;10:1371. doi: 10.2217/nnm.15.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camci-Unal G, Annabi N, Dokmeci MR, Liao R, Khademhosseini A. NPG Asia Mater. 2014;6:e99. [Google Scholar]
- 78.Xu Y, Patnaik S, Guo X, Li Z, Lo W, Butler R, Claude A, Liu Z, Zhang G, Liao J, Anderson PM, Guan J. Acta Biomater. 2014;10:3449. doi: 10.1016/j.actbio.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimizu T, Sekine H, Isoi Y, Yamato M, Kikuchi A, Okano T. Tissue Eng. 2006;12:499. doi: 10.1089/ten.2006.12.499. [DOI] [PubMed] [Google Scholar]
- 80.da Silva RMP, Mano JF, Reis RL. Trends Biotechnol. 2007;25:577. doi: 10.1016/j.tibtech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu T. In: Myocardial Tissue Engineering, Tissue Engineering for Tissue and Organ Regeneration. 1st. Eeberli DP, editor. China: InTech; 2011. p. 1. [Google Scholar]
- 82.Shimizu T, Yamato M, Kikuchi A, Okano T. Biomaterials. 2003;24:2309. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 83.Bursac N. Eng. Med. Biol. Mag., IEEE. 2009;28:80. doi: 10.1109/MEMB.2009.931792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sekiya N, Tobita K, Beckman S, Okada M, Gharaibeh B, Sawa Y, Kormos RL, Huard J. Mol. Ther. 2013;21:662. doi: 10.1038/mt.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishida O, Hagino I, Nagaya N, Shimizu T, Okano T, Sawa Y, Mori H, Yagihara T. Transl. Res. 2015;165:631. doi: 10.1016/j.trsl.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Petsche Connell J, Camci-Unal G, Khademhosseini A, Jacot JG. Tissue Eng. Part B: Rev. 2013;19:368. doi: 10.1089/ten.teb.2012.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hasan A, Paul A, Memic A, Khademhosseini A. Biomed. Microdevices. 2015;17:1. doi: 10.1007/s10544-015-9993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VT, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Tim DS, Khademhosseini A. ACS Nano. 2018;8:8050. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Z, Guo X, Palmer AF, Das H, Guan J. Acta Biomater. 2012;8:3586. doi: 10.1016/j.actbio.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 90.Nunes SS, Song H, Chiang CK, Radisic M. J. Cardiovasc. Transl. Res. 2011;4:592. doi: 10.1007/s12265-011-9307-x. [DOI] [PubMed] [Google Scholar]
- 91.Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Acta Biomater. 2014;10:11. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Tissue Eng. 2002;8:175. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 93.Chen G, Ushida T, Tateishi T. Macromol. Biosci. 2002;2:67. [Google Scholar]
- 94.Davidenko N, Gibb T, Schuster C, Best SM, Campbell JJ, Watson CJ, Cameron RE. Acta Biomater. 2012;8:667. doi: 10.1016/j.actbio.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 95.Shin EJ, Choi SM, Singh D, Zo SM, Lee YH, Kim JH, Han SS. Cellulose. 2014;21:3515. [Google Scholar]
- 96.Gaetani R, Feyen DAM, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PAFM, Sluijter JPG. Biomaterials. 2015;61:339. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Joanne P, Kitsara M, Boitard S-E, Naemetalla H, Vanneaux V, Pernot M, Larghero J, Forest P, Chen Y, Menasché P, Agbulut O. Biomaterials. 2016;80:157. doi: 10.1016/j.biomaterials.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 98.Hufenus R, Reifler FA, Maniura-Weber K, Spierings A, Zinn M. Macromol. Mater. Eng. 2012;297:75. [Google Scholar]
- 99.Herrmann FE, Lehner A, Hollweck T, Haas U, Fano C, Fehrenbach D, Kozlik-Feldmann R, Wintermantel E, Eissner G, Hagl C, Akra B. J. Biomed. Mater. Res. A. 2014;102:958. doi: 10.1002/jbm.a.34786. [DOI] [PubMed] [Google Scholar]
- 100.Fromstein JD, Zandstra PW, Alperin C, Rockwood D, Rabolt JF, Woodhouse KA. Tissue Eng. Part A. 2008;14:369. doi: 10.1089/tea.2006.0410. [DOI] [PubMed] [Google Scholar]
- 101.Rai R, Tallawi M, Barbani N, Frati C, Madeddu D, Cavalli S, Graiani G, Quaini F, Roether JA, Schubert DW, Rosellini E, Boccaccini AR. Mater. Sci. Eng. C. 2013;33:3677. doi: 10.1016/j.msec.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 102.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. Int. J. Cardiol. 2013;167:1461. doi: 10.1016/j.ijcard.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 103.Prabhakaran MP, Mobarakeh LG, Kai D, Karbalaie K, Nasr-Esfahani MH, Ramakrishna S. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2014;102:447. doi: 10.1002/jbm.b.33022. [DOI] [PubMed] [Google Scholar]
- 104.Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM. Biomaterials. 2010;31:5266. doi: 10.1016/j.biomaterials.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lo CY, Weil BR, Palka BA, Momeni A, Canty JM, Jr, Neelamegham S. Biomaterials. 2016;74:19. doi: 10.1016/j.biomaterials.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Campbell N, Suzuki K. J. Cardiovasc. Trans. Res. 2012;5:713. doi: 10.1007/s12265-012-9378-3. [DOI] [PubMed] [Google Scholar]
- 107.Cheng H, Byrska-Bishop M, Zhang CT, Kastrup CJ, Hwang NS, Tai AK, Lee WW, Xu X, Nahrendorf M, Langer R, Anderson DG. Biomaterials. 2012;33:5004. doi: 10.1016/j.biomaterials.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo CY, Antonopoulos A, Dell A, Haslam SM, Lee T, Neelamegham S. Biomaterials. 2013;34:8213. doi: 10.1016/j.biomaterials.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garbern Jessica C, Lee Richard T. Cell Stem Cell. 2013;12:689. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hernández RM, Orive G, Murua A, Pedraz JL. Adv. Drug Deliv. Rev. 2010;62:711. doi: 10.1016/j.addr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Blocki A, Beyer S, Dewavrin J-Y, Goralczyk A, Wang Y, Peh P, Ng M, Moonshi SS, Vuddagiri S, Raghunath M, Martinez EC, Bhakoo KK. Biomaterials. 2015;53:12. doi: 10.1016/j.biomaterials.2015.02.075. [DOI] [PubMed] [Google Scholar]
- 112.Lee YS, Lim KS, Oh J-E, Yoon AR, Joo WS, Kim HS, Yun C-O, Kim SW. J. Control. Rel. 2015;205:128. doi: 10.1016/j.jconrel.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paul A, Shao W, Abbasi S, Shum-Tim D, Prakash S. Mol. Pharm. 2012;9:2479. doi: 10.1021/mp3000502. [DOI] [PubMed] [Google Scholar]
- 114.Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam B-K, Ruel M, Suuronen EJ, Courtman DW, Stewart DJ, Davis DR. Biomaterials. 2014;35:133. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paul A, Cantor A, Shum-Tim D, Prakash S. Mol. Biotechnol. 2011;48:116. doi: 10.1007/s12033-010-9352-8. [DOI] [PubMed] [Google Scholar]
- 116.Hasan A, Nurunnabi M, Morshed M, Paul A, Polini A, Kuila T, Hariri MA, Lee YK, Jaffa AA. BioMed research international. 2014:18. doi: 10.1155/2014/307519. Article ID 307519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pacelli S, Manoharan V, Desalvo A, Lomis N, Jodha KS, Prakash S, Paul A. J. Mater. Chem. B. 2016 doi: 10.1039/C5TB01686J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, Black 3rd LD. Acta Biomater. 2015;14:84. doi: 10.1016/j.actbio.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sreerekha PR, Menon D, Nair SV, Chennazhi KP. Tissue Eng. Part A. 2012;19:849. doi: 10.1089/ten.TEA.2012.0374. [DOI] [PubMed] [Google Scholar]
- 120.Muscari C, Giordano E, Bonafè F, Govoni M, Guarnieri C. Stem Cells Int. 2014;2014 doi: 10.1155/2014/434169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Emmert MY, Hitchcock RW, Hoerstrup SP. Adv. Drug Deliv. Rev. 2014;69–70:254. doi: 10.1016/j.addr.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 122.Guo X-M, Zhao Y-S, Chang H-X, Wang C-Y, E L-L, Zhang X-A, Duan C-M, Dong L-Z, Jiang H, Li J, Song Y, Yang X. Circulation. 2006;113:2229. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- 123.Guan J, Wang F, Li Z, Chen J, Guo X, Liao J, Moldovan NI. Biomaterials. 2011;32:5568. doi: 10.1016/j.biomaterials.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steinhaus B, Garcia ML, Shen AQ, Angenent LT. Appl. Environ. Microbiol. 2007;73:1653. doi: 10.1128/AEM.01827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ito H, Takayanagi K, Okochi M, Shikida M, Sato K, Honda H. J. Chem. Eng. Jpn. 2006;39:1296. [Google Scholar]
- 126.Poon C, Boughton P, Ruys AJ. Conf Proc IEEE Eng Med Biol Soc. 2013:6224–6227. doi: 10.1109/EMBC.2013.6610975. [DOI] [PubMed] [Google Scholar]
- 127.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Tissue Eng. Part B, Rev. 2010;16:169. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Inamdar NK, Borenstein JT. Curr. Opin. Biotechnol. 2011;22:681. doi: 10.1016/j.copbio.2011.05.512. [DOI] [PubMed] [Google Scholar]
- 129.Montgomery M, Zhang B, Radisic M. J. Cardiovasc. Pharmacol. Therap. 2014;19:382. doi: 10.1177/1074248414528576. [DOI] [PubMed] [Google Scholar]
- 130.Hasan A, Paul A, Vrana NE, Zhao X, Memic A, Hwang Y-S, Dokmeci MR, Khademhosseini A. Biomaterials. 2014;35:7308. doi: 10.1016/j.biomaterials.2014.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsang K, Annabi N, Ercole F, Zhou K, Karst DJ, Li F, Haynes JM, Evans RA, Thissen H, Khademhosseini A. Adv. Funct. Mater. 2015;25:977. doi: 10.1002/adfm.201403124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giridharan GA, Nguyen MD, Estrada R, Parichehreh V, Hamid T, Ismahil MA, Prabhu SD, Sethu P. Anal. Chem. 2010;82:7581. doi: 10.1021/ac1012893. [DOI] [PubMed] [Google Scholar]
- 133.Hwang Y-S, Chung BG, Ortmann D, Hattori N, Moeller H-C, Khademhosseini A. Proc. Natl. Acad. Sci. 2009;106:16978. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nguyen Doan C, Hookway Tracy A, Wu Q, Jha R, Preininger Marcela K, Chen X, Easley Charles A, Spearman P, Deshpande Shriprasad R, Maher K, Wagner Mary B, McDevitt Todd C, Xu C. Stem Cell Rep. 2014;3:260. doi: 10.1016/j.stemcr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Annabi N, Selimovic S, Acevedo Cox JP, Ribas J, Afshar Bakooshli M, Heintze D, Weiss AS, Cropek D, Khademhosseini A. Lab on a Chip. 2013;13:3569. doi: 10.1039/c3lc50252j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Freed LE, Engelmayr GC, Jr, Borenstein JT, Moutos FT, Guilak F. Adv. Mater. 2009;21:3410. doi: 10.1002/adma.200900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye X, Lu L, Kolewe ME, Park H, Larson BL, Kim ES, Freed LE. Biomaterials. 2013;34:10007. doi: 10.1016/j.biomaterials.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nguyen DC, Hookway TA, Wu Q, Jha R, Preininger MK, Chen X, Easley CA, Spearman P, Deshpande SR, Maher K. Stem Cell Rep. 2014;3:260. doi: 10.1016/j.stemcr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones PH, Watt FM. Cell. 1993;73:713. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 140.Liu H-B, Gong Y-F, Yu C-J, Sun Y-Y, Li X-Y, Zhao D, Zhang Z-R. Regenerat. Med. Res. 2013;1:9. doi: 10.1186/2050-490X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barbash I, Chouraqui P, Baron J, Feinberg M, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes L, Kloner R, Leor J. Circulation. 2003;108:863. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 142.Kurdi M, Chidiac R, Hoemann C, Zouein F, Zgheib C, Booz GW. Congest. Heart Fail. 2010;16:132. doi: 10.1111/j.1751-7133.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barron V, Lyons E, Stenson-Cox C, McHugh PE, Pandit A. Ann. Biomed. Eng. 2003;31:1017. doi: 10.1114/1.1603260. [DOI] [PubMed] [Google Scholar]
- 144.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H168. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 145.Hasan A, Ragaert K, Swieszkowski W, Selimović S, Paul A, Camci-Unal G, Mofrad MRK, Khademhosseini A. J. Biomechanics. 2014;47:1949. doi: 10.1016/j.jbiomech.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 146.Barthes J, Özçelik H, Hindié M, Ndreu-Halili A, Hasan A, Engin Vrana N. BioMed Res Int. 2014 doi: 10.1155/2014/921905. Article ID 921905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Waters R, Pacelli S, Maloney R, Medhi I, Ahmed RPH, Paul A. Nanoscale. 2016 doi: 10.1039/c5nr07806g. [DOI] [PMC free article] [PubMed] [Google Scholar]