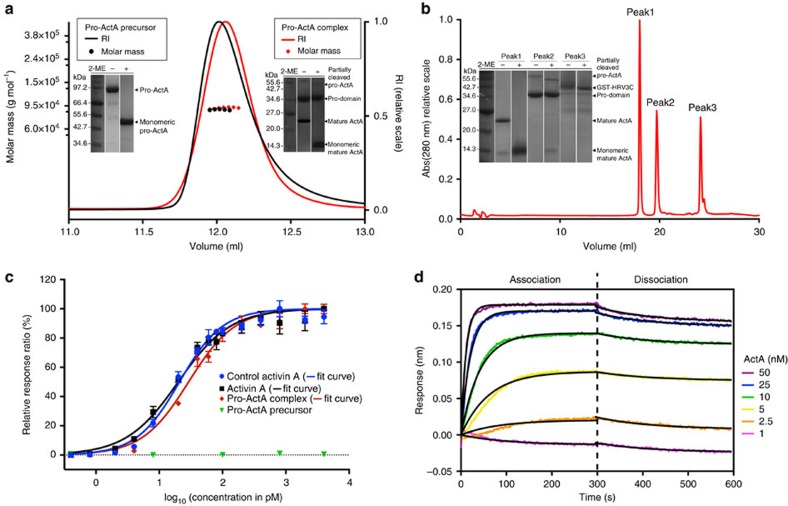
Figure 1. Characterization of pro-activin A and its activation.
(a) SEC-MALS analysis of pro-activin A. Size exclusion chromatogram of purified uncleaved pro-activin A (pro-ActA) precursor (black line) and cleaved pro-mature complex (red line) and molecular weight analysis (similarly coloured diamonds) from light scattering for the top of the eluted peak with molar mass scale shown in the left Y axis and refractive index (RI) values in the right Y axis. Peak fractions of each sample are analysed under reducing and non-reducing conditions using SDS–PAGE, shown in the inset (lanes of samples without protein have been removed from the figure). (b) Separation of pro- and mature domains of cleaved pro-activin A using reverse phase chromatography. SDS–PAGE analysis of the three peaks is shown in the inset, both under reducing and non-reducing conditions (lanes of samples without protein have been removed from the figure). (c) Bioactivity assay of activins. Analysis of bioactivity of control activin A (blue markers and dose response curve), mature activin A purified as in b (black), pro-activin A complex (red) and uncleaved pro-activin A precursor (green) using activin A inducible luciferase assay. Data are average of three replicates, normalized to Renilla luciferase and scaled to 100% of maximum signal. The error bar shows the s.d. of each data point. (d) BLI analysis of pro-domain binding to mature activin A. Coloured curves show the raw data with the concentration of activin A shown at the side of the graph. The black lines show the fitted data.