Significance
The malaria parasite requires the formation of a stage-specific, enigmatic organelle—the crystalloid—for mosquito invasion. Perhaps acting as storage for proteins and lipids, the crystalloid is generated during ookinete formation and dissolved during sporogony in the oocyst. We show here that Plasmodium berghei expresses an S-acyl-transferase DHHC10 that localizes to the crystalloid. Parasites depleted of dhhc10 produce ookinetes that successfully establish oocysts but fail to produce sporozoites; knockout parasites are characterized by the absence of the crystalloid and the mislocalization of a crystalloid-resident protein.
Keywords: palmitoylation, crystalloid, malaria, DOZI, ookinete
Abstract
Transmission of the malaria parasite from the mammalian host to the mosquito vector requires the formation of adequately adapted parasite forms and stage-specific organelles. Here we show that formation of the crystalloid—a unique and short-lived organelle of the Plasmodium ookinete and oocyst stage required for sporogony—is dependent on the precisely timed expression of the S-acyl-transferase DHHC10. DHHC10, translationally repressed in female Plasmodium berghei gametocytes, is activated translationally during ookinete formation, where the protein is essential for the formation of the crystalloid, the correct targeting of crystalloid-resident protein LAP2, and malaria parasite transmission.
The malaria parasite is capable of infecting both the vertebrate host and mosquito vector. After a mosquito blood meal, sexual precursor cells rapidly differentiate into mature gametes. In the mosquito midgut, the gametes mate to form a zygote that develops further into the motile ookinete. After crossing the midgut epithelium and establishing a sessile oocyst, the ookinete gives rise to thousands of sporozoites capable of infecting a subsequent mammalian host (1).
Sharing key organelles like the nucleus, endoplasmic reticulum, Golgi, and mitochondria with other eukaryotes, this parasite has evolved specialized, stage-specific structures that are necessary for developmental progression during parasite transmission. These include, for example, osmiophilic bodies (secretory vesicles) that release protein factors capable of lysing the parasitophorous vacuole and erythrocyte membranes, thus producing free gametes (2) and a gliding motility motor anchored to the inner membrane complex (IMC), allowing the ookinete to migrate across the mosquito midgut epithelium and establish an oocyst (3). Sporozoite formation in the oocyst finally requires the presence of a stage-specific organelle, the crystalloid, a multivesicular structure assembled in the ookinete and putative reservoir of proteins and lipids used during sporogony. Although this enigmatic organelle was discovered more than 40 y ago, its formation and function remain largely unknown (4–9). Six LCCL proteins have been shown to reside within (9) and maintain the stability (8, 9) of these organelles essential for sporogony (10).
The morphological changes taking place during zygote-to-ookinete development and the generation of thousands of sporozoites inside a single oocyst require extensive protein translation and membrane biogenesis to support the formation of organelles and plasma membrane (PM) surrounding each new parasite. One-third of the proteins identified in the oocyst and oocyst-derived (midgut) sporozoites of the human parasite Plasmodium falciparum are putatively membrane-bound (11). The targeting of such proteins to organelles, and perhaps formation of certain organelles per se, requires appropriate sorting signals, along with transmembrane (TM) domains to keep these factors in place. Posttranslational modifications, such as lipidation, can increase the affinity of a modified protein for membranes and alter its subcellular localization. Only palmitoylation—the addition of a C-16 long-chain fatty acid to a cysteine residue—is reversible and thus able to dynamically influence protein–protein interactions, function, and gene expression (12–17).
Catalyzed by TM-spanning enzymes known as palmitoyl-S-acyl-transferases (DHHC-PATs; PATs) this posttranslational modification is evolutionarily conserved; 25 PATs are known in humans, 7 in Saccharomyces cerevisiae, 12 in the human malaria parasite P. falciparum, and 11 in the rodent malaria parasite Plasmodium berghei. In asexual blood stages of P. falciparum, several hundred proteins have been found to be palmitoylated, and the addition of the inhibitor 2-bromopalmitate (2-BMP) to P. falciparum in vitro cultures is known to cause developmental as well as red blood cell invasion defects in schizonts, the latter likely through the destabilization of gliding motility motor components (15). In P. berghei, the inhibitor causes severe ookinete formation defects (18).
Despite the known localization data for several Plasmodium PATs (14, 17, 19), the specific functions of individual S-acyl-transferases for life cycle progression are almost completely unknown; only recently has DHHC2 been identified as being required for ookinete morphogenesis, specifically zygote elongation, and as having a likely essential role in blood stage parasite development (18). In the present work, we provide conclusive genetic evidence of the essential role of the stage-specific S-acyl-transferase DHHC10 for mosquito infection by P. berghei. Here dhhc10 is maternally provided as a translationally repressed messenger ribonucleoprotein (mRNP) to the developing ookinete by the female gametocyte. In the ookinete, the protein controls formation of the crystalloid, and ultimately guarantees the success of sporozoite formation and transmission to a subsequent host.
Results
DHHC10 Is Required for Mosquito Infection.
Palmitoylation is crucial for P. falciparum schizogony and P. berghei ookinete formation (15, 18). Here we investigated the role of a maternally provided, translationally repressed S-acyl-transferase for developmental progression in the mosquito vector. Following a mosquito blood meal, mating of males and females produces the round zygote that develops into a motile, banana-shaped ookinete. This transformation process relies heavily on translationally repressed (stored) mRNAs provided by the female gametocyte (20–22). Of 11 annotated P. berghei DHHC-PATs (17, 23) we have identified dhhc10, along with dhhc2 and dhhc3, as being up-regulated in gametocytes (Fig. 1A) (24). Whereas dhhc2 (18) and dhhc3 expression was also evident in asexual blood stage forms, only dhhc10 was exclusively transcribed in sexual stages (Fig. 1B), suggesting a role for the protein during transmission of gametocytes to the mosquito vector. DHHC10 is 275-aa long and has four distinct transmembrane domains (www.cbs.dtu.dk/services/TMHMM/), a feature it shares with the syntenic orthologs from P. falciparum and Plasmodium vivax, two major malaria parasite species that infect humans (Fig. 1C and SI Appendix, Fig. S1).
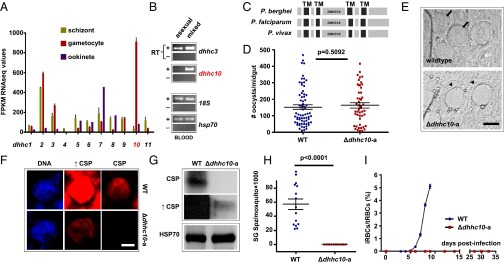
Fig. 1.
DHHC10 is essential for transmission to the mosquito vector. (A) RNA sequencing data from blood and mosquito stages for all 11 P. berghei dhhc genes reveal that dhhc2, dhhc3, and dhhc10 are up-regulated in gametocytes (24). Error bars correspond to SEM values. FPKM, fragments per kilobase of transcript per million mapped reads. (B) RT-PCR of dhhc3 and dhhc10 confirm the gametocyte-specific nature of dhhc10, whereas dhhc3 is also transcribed in asexual stage parasites. RT+, RT-positive reaction; RT−, RT-negative reaction. (C) Schematic representation of rodent and human malaria DHHC10 with four TM domains. The signature DHHC motif is located between TM domains 2 and 3. Drawn to scale. (D) Δdhhc10-a parasites develop WT oocyst numbers at day 12–13 postinfection. Absolute numbers of oocysts/midgut from five independent experiments are presented for both WT (n = 70) and Δdhhc10-a (n = 48) parasites. Mean ± SEM values are shown; P values are for the Mann–Whitney test. (E) Δdhhc10-a oocysts appear normal-sized at day 14 postinfection but lack signs of sporulation and remain empty (arrowheads), whereas WT oocysts have already formed sporozoites (arrows). (Scale bar: 20 µm.) (F) Immunofluorescence of oocyst-infected midguts at day 14 postinfection reveals strongly reduced expression of the developmental marker CSP in Δdhhc10-a parasites; DNA staining (Hoechst 33342) is comparable. ↑CSP indicates long exposure. (Scale bar: 20 µm.) (G) Western blot analyses of oocyst-infected midguts at day 13 postinfection confirms low CSP expression in Δdhhc10-a mutants. Here HSP70 served as a loading control. ↑CSP indicates overexposure. (H) Δdhhc10-a parasites do not colonize the mosquito salivary glands. WT, five independent experiments, n = 15; Δdhhc10-a, four independent experiments, n = 10. Mean ± SEM values are shown. (I) Mice bitten by Δdhhc10-a–infected mosquitoes fail to develop blood stage infections. Mean ± SEM parasitemias from three independent experiments are shown.
Using standard methods of P. berghei genetic modification (25), we generated two independent dhhc10 null mutants, Δdhhc10-a and Δdhhc10-b (SI Appendix, Table S1 and Fig. S2). Although asexual growth and schizont formation in P. falciparum requires palmitoylation (15), our dhhc10 null mutants showed normal asexual growth and gametocyte production rates. (SI Appendix, Table S2 presents key developmental data for both independent knockout clones.) This could indicate that DHHC10 exerts a nonessential role during schizont formation (asexual development) and gametocytogenesis (intraerythrocytic sexual development), but, given its transcriptional pattern, supports the idea that DHHC10-driven palmitoylation does not occur.
To identify a possible role for DHHC10 during developmental progression and transmission (ookinete formation, establishment of oocysts, or sporogony), we allowed Anopheles stephensi mosquitoes to feed on mice infected with Δdhhc10 parasites. Two independent null mutant clones (Δdhhc10-a and Δdhhc10-b) produced numbers of oocysts similar to those seen on infection with wild type (WT) parasites (Fig. 1D and SI Appendix, Table S2), indicating that they were not compromised in their ability to form infection-competent, motile ookinetes and to establish oocysts that could resist elimination by the host’s immune response. However, although similar in size to WT oocysts, the mutants had an “empty” appearance and showed no signs of sporulation (Fig. 1E), the process during which a single oocyst gives rise to thousands of motile sporozoites. These parasites lacked appreciable expression of circumsporozoite protein (CSP), the major sporozoite surface protein and a key developmental marker of sporogony. Whereas CSP is strongly expressed in WT oocysts of the same age, immunofluorescence assays (IFAs) (Fig. 1F) and Western blot analyses (Fig. 1G) of protein extracts from pooled infected midguts showed minimal expression of CSP in the Δdhhc10 parasites, even though labeling of the same cells with the DNA-specific dye Hoechst 33342 demonstrated no defect in DNA replication (Fig. 1F). The mutants failed entirely to colonize the mosquito salivary glands (Fig. 1H and SI Appendix, Table S2) and consequently were unable to transmit the infection to naïve mice (Fig. 1I and SI Appendix, Table S2).
dhhc10 mRNA Is Maternally Supplied, Translated During Ookinete Formation, and Targeted to Crystalloids.
The ability of Δdhhc10 mutants to generate normal numbers of oocysts while failing to develop further into sporozoites was unexpected in view of the transcription profile identified by RT-PCR; mRNA was clearly detected in gametocytes (Fig. 1 A and B). To characterize DHHC10 protein expression, timing, and localization in detail, we generated a parasite expressing a GFP-tagged version of the endogenous protein (Fig. 2A and SI Appendix, Fig. S3). This mutant showed normal progression throughout the life cycle, including ookinete, oocyst, and sporozoite production and transmission to mice via mosquito bites (SI Appendix, Table S2); thus, GFP-tagging of DHHC10 did not affect protein function or parasite viability.
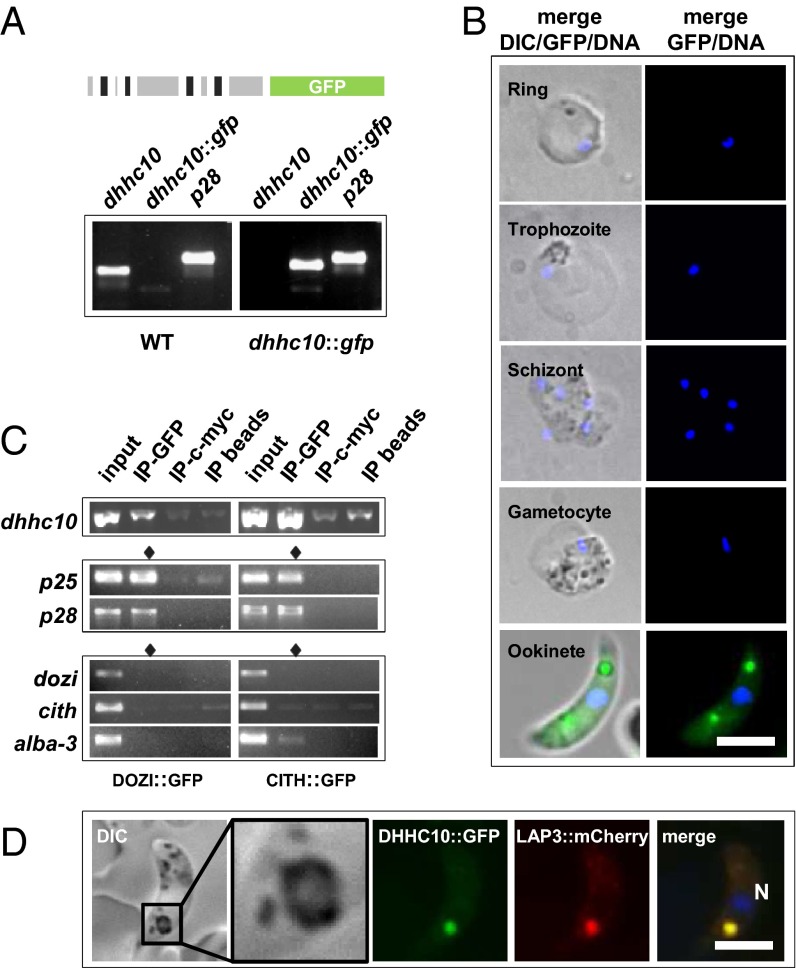
Fig. 2.
dhhc10 is translationally repressed in gametocytes and translated in ookinetes, where it localizes to the crystalloid. (A) Only dhhc10::gfp mRNA is transcribed in dhhc10::gfp parasites. p28 served as the control gene for RT-PCR. (B) DHHC10::GFP is translated only in ookinetes. DNA was stained with Hoechst 33342. (Scale bar: 5 µm.) (C) RT-PCR analyses of DOZI::GFP and CITH::GFP gametocyte IP eluates showing that dhhc10 mRNA, like p25 and p28 mRNAs, is bound by both translational repressors. Translated mRNAs (dozi, cith, and alba-3) are not enriched in the IP-GFP fractions. Input, total gametocyte mRNA; IP-GFP, IP with anti-GFP antibody; IP-c-myc, IP with anti–c-myc antibody; IP beads, no antibody used for IP. (D) DHHC10::GFP colocalizes with the crystalloid-resident protein LAP3 (here tagged with mCherry). (Inset) A higher-magnification view of the crystalloid as seen on DIC microscopy. N, nucleus stained with Hoechst 33342. (Scale bar: 5 µm.)
As expected from the RT-PCR results, we found no protein expression in asexual stage parasites; in addition, DHHC10::GFP was not detected in gametocytes until being translated in the ookinete (Fig. 2B and SI Appendix, Fig. S4), a behavior consistent with translational regulation during the transmission of P. berghei found for many genes (20–22). Storage of this mRNA in cytoplasmic mRNPs for translation in the ookinete was confirmed in an RNA immunoprecipitation (IP) experiment, in which dhhc10 was copurified with the translational repressors DOZI (a homolog of yeast DHH1p) and CITH (a homolog of yeast Scd6p) (Fig. 2C), similar to two well-characterized translationally repressed mRNAs encoding the ookinete surface proteins P25 and P28 (20–22). Failure to store translationally repressed mRNAs in the absence of DOZI and CITH affects female fertility, but not male fertility (20–22).
In ookinetes, DHHC10::GFP highlighted distinct cytoplasmic foci (Fig. 2B and SI Appendix, Fig. S4) known for members of the LAP/CCp protein family typical for the ookinete/oocyst-specific crystalloid organelle (8, 26, 27). Believed to act as a reservoir for proteins required for sporozoite development within the oocyst, these organelles are generated in ookinetes but dissolve in early oocysts and are absent in late oocysts (6, 9). Focal localization of DHHC10::GFP in one or two spots was observed in ∼75% of ookinetes (n = 60). The remaining 25% showed a somewhat more diffuse GFP signal throughout the ookinete cytoplasm; dim and diffuse fluorescence was also present in ookinetes that exhibited clear DHHC10::GFP foci (SI Appendix, Fig. S4).
To corroborate the targeting of DHHC10 to crystalloid bodies, we transfected dhhc10::gfp parasites with a plasmid construct encoding a C-terminally mCherry-tagged version of LAP3 (SI Appendix, Fig. S5). This plasmid contains lap3 upstream regulatory regions, allowing for the episomal expression of LAP3::mCherry under its endogenous promoter. LAP3 has previously been localized to the ookinete crystalloids by both live fluorescence imaging and immunoelectron microscopy (27). DHHC10 perfectly colocalized with LAP3 in these experiments, unambiguously identifying this PAT in the ookinete crystalloid (Fig. 2D). In the differential interference contrast (DIC) image, the crystalloid is seen surrounded by hemozoin, the remnant of hemoglobin digestion from the intraerythrocytic female gametocyte and also known as malaria pigment (Fig. 2D).
Crystalloids are formed by the microtubule (MT)-dependent transport and assembly of endoplasmic reticulum-derived vesicles (9). Concomitantly, LAP proteins such as LAP3 are shuttled to common assembly points and incorporated into mature crystalloids. We took advantage of the dhhc10::gfp;lap3::mCherry line to closely follow DHHC10 translation and trafficking in relation to LAP3. DHHC10::GFP was detected as early as 3 h postfertilization, and both DHHC10 and LAP3 showed signs of protein concentration by 9 h postfertilization. At 12 h, more than 50% of the retorts displayed focal accumulation of both proteins, and by the end of ookinete development (24 h), GFP and mCherry signals colocalized in the crystalloid in more than 90% of mature ookinetes (SI Appendix, Fig. S6). Thus, no significant differences between the two proteins in either the timing of expression or subcellular localization were evident.
DHHC10 Is Required for Crystalloid Formation.
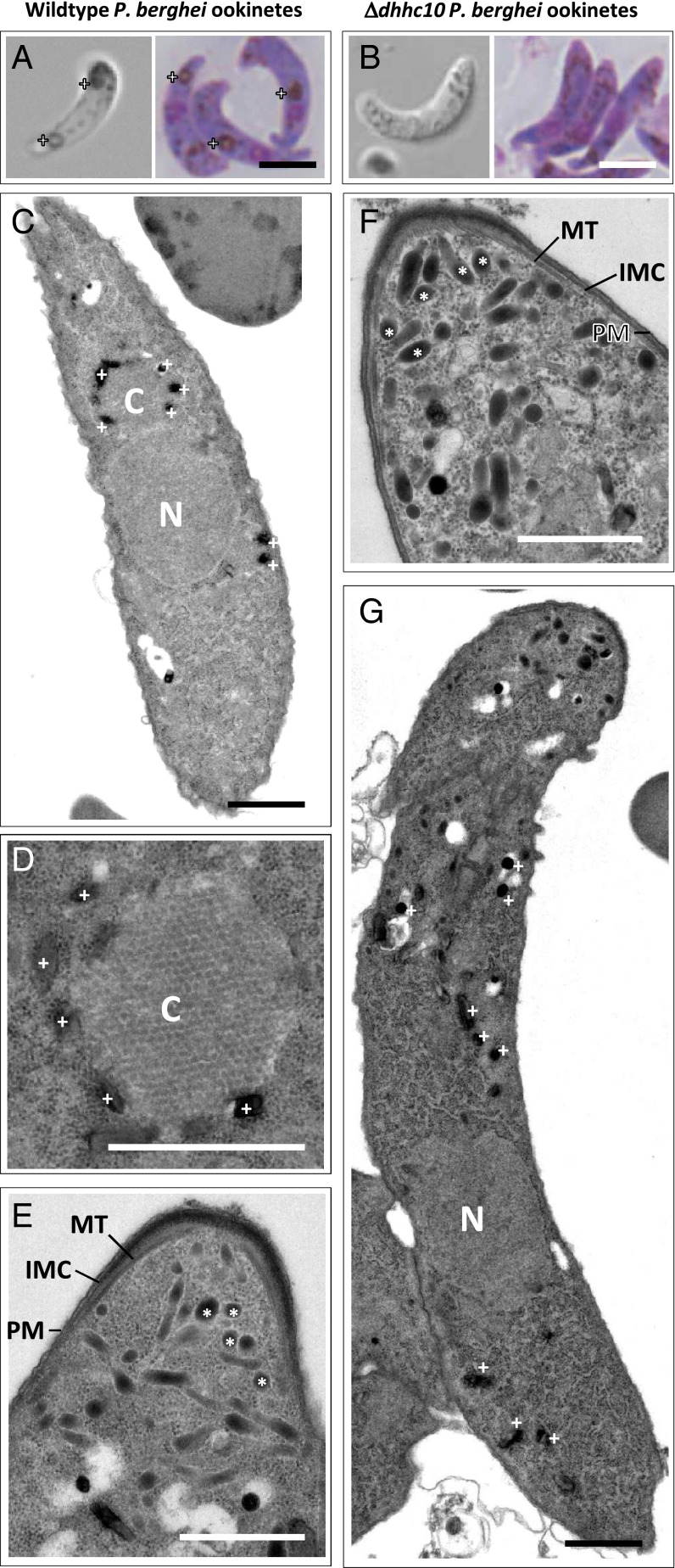
In vivo, Δdhhc10 mutants produced WT numbers of oocysts, whereas in vitro they showed normal ookinete formation rates (SI Appendix, Table S2). Compared with WT ookinetes (Fig. 3A), mutant ookinetes displayed a normal shape and efficiently invaded mosquito midguts, but lacked the hemozoin clusters (Fig. 3B) that typically surround and highlight crystalloids in WT P. berghei ookinetes (4, 28); they were dispersed throughout the cytoplasm in live and fixed, Giemsa-stained cells (Fig. 3B). To unequivocally confirm the absence of this organelle, we compared WT and Δdhhc10 mutants by transmission electron microscopy (TEM). In 50% of the WT ookinetes (6 of 12), we were able to visualize the crystalloid, which appeared as a highly ordered (almost crystalline) structure surrounded by malaria pigment (Fig. 3 C and D). The crystalloid was located close to the nucleus, often posterior to the apical complex, which harbors the micronemes (Fig. 3E). Micronemes are secretory vesicles that provide adhesive proteins required for gliding motility and invasion of the midgut epithelium. The apical complex contains the gliding motility motor (3), which is anchored to the IMC between the PM and the subpellicular MTs (Fig. 3E). Micronemes were clearly visible in the knockout mutant, and the organization of the IMC and the subpellicular network appeared normal (Fig. 3F), explaining the WT invasion behavior in the in vivo transmission experiments (Fig. 1D and SI Appendix, Table S2). In contrast, in all 30 Δdhhc10 ookinetes examined, we failed to localize characteristic crystalloids and found hemozoin scattered throughout the cytoplasm (Fig. 3G).
Fig. 3.
DHHC10 is required for crystalloid formation. (A) WT ookinetes with crystalloids (+) under DIC microscopy (live) and after Giemsa staining (fixed). (Scale bar: 5 µm.) (B) Δdhhc10-a ookinetes show no evidence of crystalloid formation. (Scale bar: 5 µm.) (C) Longitudinal TEM section of a WT ookinete. N, nucleus; C, crystalloid surrounded by hemozoin (+). (Scale bar: 1 µm.) (D) TEM of WT crystalloid (C) reveals an ordered, honeycomb arrangement surrounded by hemozoin (+). (Scale bar: 1 µm.) (E) Apical complex of a WT ookinete with micronemes (*) and a typical arrangement of subpellicular MT, IMC, and PM. (Scale bar: 1 µm.) (F) Apical complex of Δdhhc10-b ookinetes showing no affect on micronemes (*) or MT, IMC, and PM arrangement. (Scale bar: 1 µm.) (G) Longitudinal TEM section of a Δdhhc10-b ookinete highlights the nucleus (N), but does not reveal a distinct crystalloid. Hemozoin (+) is scattered throughout the cytoplasm. (Scale bar: 1 µm.)
Highly down-regulated in dozi null mutants (21), dhhc10 mRNA is likely transcribed and provided to developing zygotes solely by females. To test this, we crossed Δdhhc10 parasites with the Δp47 parasite line, a mutant producing fertile males but sterile females (29). The resulting ookinetes lacked crystalloids, whereas ookinetes from the control experiment—crossing Δp47 males with fertile females from line Δp48/45 (30)—did not (SI Appendix, Fig. S7). Fertilization rates were similar in the two crosses (SI Appendix, Fig. S7), whereas no fertilization occurred in the Δp47 or the Δp48/45 parasites. The Δdhhc10/Δp47 cross did not rescue the crystalloid formation defect of Δdhhc10 ookinetes (SI Appendix, Fig. S7), confirming that the WT dhhc10 allele of the male Δp47 gene does not allow for proper crystalloid biogenesis. Thus, DHHC10 expression in ookinetes results from translationally repressed DOZI- and CITH-associated dhhc10 mRNA provided by the female gamete.
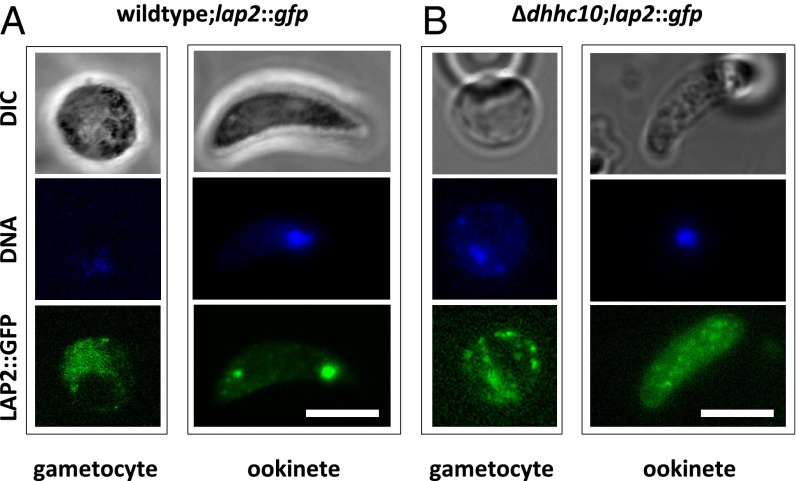
To identify the consequences of crystalloid absence in Δdhhc10 ookinetes for crystalloid-resident proteins, we tagged LAP2 with GFP in the WT and Δdhhc10 null mutant genetic backgrounds (27). P. berghei LAP2 is a member of the LCCL multigene family of proteins expressed in female gametocytes with a typically scattered staining pattern in the WT background; during ookinete formation, it is concentrated in the crystalloid (27) (Fig. 4A). The resulting Δdhhc10;lap2::gfp parasites (SI Appendix, Fig. S8) also showed LAP2::GFP-positive female gametocytes with protein dispersed throughout the cytoplasm, as described previously (27). Whereas in WT ookinetes, LAP2::GFP is targeted to the crystalloids, the absence of DHHC10 left LAP2::GFP protein scattered throughout the cell (Fig. 4B).
Fig. 4.
Absence of DHHC10 disturbs localization of crystalloid-resident protein LAP2. Live imaging of lap2::gfp (A) and Δdhhc10;lap2::gfp (B) female gametocytes shows LAP2::GFP expression throughout the gametocyte cytoplasm in both strains. Only in WT ookinetes is LAP2::GFP trafficked to crystalloids, whereas in Δdhhc10;lap2::gfp parasites, the protein remains distributed throughout the ookinete cytoplasm. DNA was stained with Hoechst 33342. (Scale bars: 5 µm.)
To investigate how the lack of DHHC10 affects crystalloid formation during zygote-to-ookinete transformation, we imaged Δdhhc10;lap2::gfp parasites in a time-course experiment. More than 50 parasites were imaged at each time point, and in all developmental stages, the GFP signal showed an exclusively diffuse cytoplasmic location with no evidence of LAP2::GFP clustering or hemozoin crystals as seen on microscopic analysis of live or fixed, Giemsa-stained parasites. Instead, small hemozoin crystals remained scattered throughout the cytoplasm (SI Appendix, Fig. S9). These results strongly support the conclusion that crystalloid formation is abrogated in the absence of DHHC10. As a result, crystalloid-resident proteins remain localized in the cytoplasm. However, the absence of crystalloid assembly also could be a consequence of mistrafficking of nonpalmitoylated LAP2 itself. Whether LAPs are palmitoylated is unknown, however.
Finally, to unequivocally attribute the lack of crystalloid formation and protein targeting to this organelle to the absence of DHHC10, we complemented the Δdhhc10-a parasite line with dhhc10::gfp (SI Appendix, Fig. S10). In the clonal line Δdhhc10;dhhc10::gfp, we readily observed the presence of DHHC10::GFP in bright fluorescent spots in mature ookinetes (SI Appendix, Fig. S11), characteristic of crystalloids as shown in dhhc10::gfp ookinetes (Fig. 2B and SI Appendix, Fig. S4). Clustered hemozoin crystals were visible under DIC imaging, indicating the normal formation of crystalloids. These results show that the introduction of a WT copy of dhhc10 into the genome of Δdhhc10 parasites rescued crystalloid formation.
Discussion
This study highlights the essential nature of the S-acyltransferase DHHC10 in malaria parasite life cycle progression in the mosquito vector. We have shown that DHHC10 is essential for sporozoite formation, a prerequisite for malaria transmission to the subsequent mammalian host. Although transcribed and translationally repressed in the gametocyte, DHHC10 plays no immediate role in the development of gametes or ookinetes. Gametes are fertile, form zygotes, and develop into ookinetes that infect the mosquito midgut epithelium in WT numbers. DHHC10 is essential for the formation of crystalloids, transient organelles of ookinetes and oocysts that exert their function in oocysts. In the absence of DHHC10, crystalloids could not be detected by light microscopy or electron microscopy, resulting in an aberrant localization of the crystalloid-resident protein LAP2. Mutants ultimately failed to produce sporozoites. Our work confirms that crystalloids are dispensable for the development of ookinetes and initial colonization of the mosquito midgut, but essential for the development of sporozoites in oocysts and thus for parasite transmission between host and vector.
Formed in ookinetes, the crystalloids persist up to the young oocyst stage (6). It has been suggested that the organelle acts as a protein (4, 31) or a lipoprotein-energy reservoir (7) during oocyst development and sporozoite formation (5, 6). The crystalloid appears as a prominent honeycomb-patterned structure believed to consist of individual vesicles that traffic or provide cargo to their destination during sporogony. That this organelle is likely membranous in nature is highlighted by the localization of the four-transmembrane domain protein DHHC10::GFP to this structure. How the regular shape of the crystalloid is established or maintained is unclear; perhaps palmitoylation of crystalloid-resident proteins helps organize, maintain, and tether individual vesicles together in this typical arrangement. As illustrated by the Δdhhc10;lap2::gfp parasites, the crystalloid-resident protein LAP2 is not degraded in the mutant. The protein either fails to be trafficked to and be concentrated in the now-absent crystalloid or requires DHHC10-mediated palmitoylation to promote crystalloid formation. Therefore, the proper expression and packaging of such components is important for sporozoite development. Lack of crystalloid formation also has been reported for null mutants of the crystalloid-resident LCCL protein PbSR (LAP1), although the block in sporogony is not absolute (8), and, more recently, also for parasites lacking LAP3 (9). Gene deletion mutants of additional LCCL/LAP protein family members (10, 32, 33), plasmepsin VI (34) and rhomboid 3 (35), which may be bona fide crystalloid residents, present similar phenotypes with respect to oocyst differentiation. In P. berghei, hemozoin-containing vacuoles accumulate around the crystalloid’s edges (4, 28). In the DHHC10 knockout, malaria pigment remained scattered throughout the cytoplasm. Whether this property is biologically relevant is unknown.
The targets of protein palmitoylation in the early mosquito stages of the malaria parasite remain to be identified, although inhibition of palmitoylation and deletion of P. berghei dhhc2 are known to severely affect zygote elongation (18). The published rodent P. berghei (36, 37) and avian Plasmodium gallinaceum (38) malaria zygote and ookinete proteomes comprehend 277 out of 1,368 (20%) P. falciparum orthologs found to be palmitoylated in P. falciparum schizonts. A comparison of oocyst and oocyst-derived sporozoite (ODS) proteome data from P. falciparum (11) with the palmitome of P. falciparum blood stage schizonts (15) showed that 54 of 134 proteins detected in 7- to 8-d-old oocysts were targets of palmitoylation in blood stages, whereas 176 of the 453 proteins detected in 13- to 14-d-old ODS were present in the schizont palmitome. Whether the enzymatic activity of DHHC10 is required for the phenotype observed here remains unclear, however.
Palmitoylation is clearly important for protein stability and trafficking (39), but enzymatic activity-independent function of the yeast palmitoyl-transferase Swf1p also has been reported (40). With poorly defined palmitoylation motifs (41), proteomic strategies (15, 42) will be required to identify the true extent of protein palmitoylation in these stages, characterize their effect on parasite morphogenesis, and determine whether crystalloid formation and maintenance necessitates palmitoylation of crystalloid-resident proteins such as the LAPs. The identification of a clear phenotype linked to DHHC10 will help define enzyme-substrate pairs.
Our results serve as a starting point for further experiments to identify targets that require palmitoylation for proper function and formation of the Plasmodium crystalloid. Given their effect on the transmission from the mosquito vector to the mammalian host, the development of parasite-specific DHHC-PAT inhibitors will certainly be of interest for novel intervention strategies. The transcription, yet translational repression, of an mRNA (dhhc10) in the blood stage gametocyte, whose protein product directs the generation and maintenance of an organelle, the crystalloid, which per se is redundant for ookinete formation, morphology, and midgut invasion but plays an essential role in developmental progression during the next life cycle stage (oocyst sporogony) to guarantee successful transmission, is an astonishing evolutionary development.
Materials and Methods
Experimental Animals.
Female BALB/c ByJ and OF-1 (6- to 8-wk-old, bred at Charles River, France) mice were used. All animal experiments in this study were carried out in accordance with the European Guideline 86/609/EEC and followed the Federation of European Laboratory Animal Science Associations guidelines and recommendations concerning laboratory animal welfare. Experiments performed at the Leiden University Medical Center were approved by the Animal Experiments Committee of the Leiden University Medical Center (DEC 10099, 12042, and 12120). Experiments performed at the Instituto de Medicina Molecular were approved by the institute’s Animal Ethics Committee (under authorization AEC_2010_018_GM_Rdt_General_IMM) and the Portuguese authorities (Direção Geral de Alimentação e Veterinária), and were in compliance with Portuguese law (Portaria 1005/92). Experiments performed in Heidelberg were approved by the Regierungspräsidium Karlsruhe.
Reference P. berghei ANKA lines and generation of mutant parasite lines, RT-PCR, in vivo multiplication of asexual blood stages, gametocyte production and ookinete formation assays, oocyst and sporozoite production, and transmission experiments are described in SI Appendix, SI Materials and Methods.
Live Imaging and IFA of Blood Stages, Ookinetes, and Oocysts.
Live imaging of transgenic parasites expressing GFP-tagged DHHC10, GFP-tagged LAP2, and LAP3::mCherry was done by collecting tail blood samples from infected mice and samples from ookinete cultures at different time points after gametocyte activation and staining with 1 µg/mL Hoechst 33342/PBS. For detection of DHHC10::GFP expression in blood stages by IFA, mouse RBCs infected with dhhc10::gfp parasites were stained with rabbit polyclonal anti-GFP (Abcam; ab6556, 1:500) as the primary antibody and goat anti-rabbit IgG-Alexa Fluor 488 (Jackson ImmunoResearch Laboratories; 111–545-003, 1:500) as the secondary antibody. To detect CSP expression in Fluo-frmg and Δdhhc10-a oocysts, parasites were stained with 3D11 mouse anti-PbCSP (43) (10 µg/mL) as the primary antibody and goat anti-mouse IgG-Cy3 (Jackson ImmunoResearch Laboratories; 115–166-003, 1:400) as the secondary antibody. In all IFAs, samples were fixed with 4% PFA/PBS for 10 min at room temperature (RT), permeabilized with 0.1–0.5% Triton X-100/PBS, and blocked for 1 h at RT with 1–3% BSA/PBS. All antibody incubations were done in blocking solution for 1 h at RT, with 1–5 µg/mL Hoechst 33342/PBS used to stain nuclei. Images were obtained with a Leica DM5000B, Leica DM IRBE, or Zeiss Axiovert 200M fluorescence microscope and processed using ImageJ 1.47n software (imagej.nih.gov/ij).
Transmission electron microscopy of ookinetes and Western blot analysis of CSP expression in Δdhhc10 oocysts are described in SI Appendix, SI Materials and Methods.
Genetic Crosses, Fertilization Rates, and Crystalloid Formation.
The fertility of WT and mutant gamete populations was analyzed by standard in vitro fertilization and ookinete maturation assays (see above). The fertilization rate, defined as the percentage of female gametes that develop into zygotes or ookinetes, was determined by counting female gametes and zygotes/ookinetes in Giemsa-stained blood smears at 16–18 h after in vitro induction of gamete formation. The fertility of individual sexes (macrogametes and microgametes) was determined by in vitro cross-fertilization studies (21, 22, 44) in which gametes were cross-fertilized with gametes of parasite lines that produce only fertile male gametes (Δp47; clone 270cl1; ref. 29) or only fertile female gametes (Δp48/45; clone 137cl7; ref. 30). Crystalloid formation was scored on the same Giemsa-stained smears used for fertilization rate determination.
Statistical Methods.
Statistical analyses of oocyst and sporozoite numbers were performed with the Mann–Whitney test using GraphPad Prism 5.0.
Supplementary Material
Acknowledgments
We thank Ana Guerreiro, Ana Parreira, Fernanda Baptista, and Johannes Kroeze for management and experimental support; Mário da Costa and Leonor Pinho for mosquito production; and António Mendes for technical advice. J.T.D. was supported by Wellcome Trust Grant 088449 and United Kingdom Biotechnology and Biological Sciences Research Council Grant BB/M001598. C.J.J. was supported by the European Community’s Seventh Framework Programme (FP7/2007–2013; no. 242095). F.F. was supported by the Chica and Heinz Schaller Foundation and a European Research Council Starting Grant. J.M.S. received a PhD fellowship grant (SFRH/BD/63849/2009) from Fundação para a Ciência e a Tecnologia (FCT). G.R.M. was supported by FCT Grants PTDC/BIA-BCM/105610/2008 and PTDC/SAU-MIC/122082/2010.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522381113/-/DCSupplemental.
References
- 1.Sinden RE. The cell biology of malaria infection of mosquito: Advances and opportunities. Cell Microbiol. 2015;17(4):451–466. doi: 10.1111/cmi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuehn A, Pradel G. The coming-out of malaria gametocytes. J Biomed Biotechnol. 2010;2010:976827. doi: 10.1155/2010/976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum J, Gilberger TW, Frischknecht F, Meissner M. Host-cell invasion by malaria parasites: Insights from Plasmodium and Toxoplasma. Trends Parasitol. 2008;24(12):557–563. doi: 10.1016/j.pt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Garnham PC, Bird RG, Baker JR, Desser SS, el-Nahal HM. Electron microscope studies on motile stages of malaria parasites, VI: The oökinete of Plasmodium berghei yoelii and its transformation into the early oöcyst. Trans R Soc Trop Med Hyg. 1969;63(2):187–194. doi: 10.1016/0035-9203(69)90145-x. [DOI] [PubMed] [Google Scholar]
- 5.Lemgruber L, Lupetti P. Crystalloid body, refractile body and virus-like particles in Apicomplexa: What is in there? Parasitology. 2012;139(3):285–293. doi: 10.1017/S0031182011002034. [DOI] [PubMed] [Google Scholar]
- 6.Dessens JT, Saeed S, Tremp AZ, Carter V. Malaria crystalloids: Specialized structures for parasite transmission? Trends Parasitol. 2011;27(3):106–110. doi: 10.1016/j.pt.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trefiak WD, Desser SS. Crystalloid inclusions in species of Leucocytozoon, Parahaemoproteus, and Plasmodium. J Protozool. 1973;20(1):73–80. doi: 10.1111/j.1550-7408.1973.tb06005.x. [DOI] [PubMed] [Google Scholar]
- 8.Carter V, Shimizu S, Arai M, Dessens JT. PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids. Mol Microbiol. 2008;68(6):1560–1569. doi: 10.1111/j.1365-2958.2008.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeed S, Tremp AZ, Dessens JT. Biogenesis of the crystalloid organelle in Plasmodium involves microtubule-dependent vesicle transport and assembly. Int J Parasitol. 2015;45(8):537–547. doi: 10.1016/j.ijpara.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raine JD, et al. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog. 2007;3(3):e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasonder E, et al. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 2008;4(10):e1000195. doi: 10.1371/journal.ppat.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairbank M, Huang K, El-Husseini A, Nabi IR. RING finger palmitoylation of the endoplasmic reticulum Gp78 E3 ubiquitin ligase. FEBS Lett. 2012;586(16):2488–2493. doi: 10.1016/j.febslet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Lakkaraju AK, et al. Palmitoylated calnexin is a key component of the ribosome-translocon complex. EMBO J. 2012;31(7):1823–1835. doi: 10.1038/emboj.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones ML, Tay CL, Rayner JC. Getting stuck in: Protein palmitoylation in Plasmodium. Trends Parasitol. 2012;28(11):496–503. doi: 10.1016/j.pt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Jones ML, Collins MO, Goulding D, Choudhary JS, Rayner JC. Analysis of protein palmitoylation reveals a pervasive role in Plasmodium development and pathogenesis. Cell Host Microbe. 2012;12(2):246–258. doi: 10.1016/j.chom.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corvi MM, Alonso AM, Caballero MC. Protein palmitoylation and pathogenesis in apicomplexan parasites. J Biomed Biotechnol. 2012;2012:483969. doi: 10.1155/2012/483969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frénal K, et al. Global analysis of apicomplexan protein S-acyl transferases reveals an enzyme essential for invasion. Traffic. 2013;14(8):895–911. doi: 10.1111/tra.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos JM, et al. The Plasmodium palmitoyl-S-acyl-transferase DHHC2 is essential for ookinete morphogenesis and malaria transmission. Sci Rep. 2015;5:16034. doi: 10.1038/srep16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wetzel J, et al. The role of palmitoylation for protein recruitment to the inner membrane complex of the malaria parasite. J Biol Chem. 2015;290(3):1712–1728. doi: 10.1074/jbc.M114.598094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerreiro A, et al. Genome-wide RIP-Chip analysis of translational repressor-bound mRNAs in the Plasmodium gametocyte. Genome Biol. 2014;15(11):493. doi: 10.1186/s13059-014-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mair GR, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313(5787):667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mair GR, et al. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010;6(2):e1000767. doi: 10.1371/journal.ppat.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurrecoechea C, et al. PlasmoDB: A functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37(Database issue):D539–D543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto TD, et al. A comprehensive evaluation of rodent malaria parasite genomes and gene expression. BMC Biol. 2014;12(1):86. doi: 10.1186/s12915-014-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc. 2006;1(1):346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 26.Saeed S, Carter V, Tremp AZ, Dessens JT. Translational repression controls temporal expression of the Plasmodium berghei LCCL protein complex. Mol Biochem Parasitol. 2013;189(1-2):38–42. doi: 10.1016/j.molbiopara.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeed S, Carter V, Tremp AZ, Dessens JT. Plasmodium berghei crystalloids contain multiple LCCL proteins. Mol Biochem Parasitol. 2010;170(1):49–53. doi: 10.1016/j.molbiopara.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinden RE, Hartley RH, Winger L. The development of Plasmodium ookinetes in vitro: An ultrastructural study including a description of meiotic division. Parasitology. 1985;91(Pt 2):227–244. doi: 10.1017/s0031182000057334. [DOI] [PubMed] [Google Scholar]
- 29.van Dijk MR, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 2010;6(4):e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dijk MR, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104(1):153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 31.Garnham PC, Bird RG, Baker JR. Electron microscope studies of motile stages of malaria parasites, III: The ookinetes of Haemamoeba and Plasmodium. Trans R Soc Trop Med Hyg. 1962;56:116–120. doi: 10.1016/0035-9203(62)90137-2. [DOI] [PubMed] [Google Scholar]
- 32.Claudianos C, et al. A malaria scavenger receptor-like protein essential for parasite development. Mol Microbiol. 2002;45(6):1473–1484. doi: 10.1046/j.1365-2958.2002.03118.x. [DOI] [PubMed] [Google Scholar]
- 33.Lavazec C, et al. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol Biochem Parasitol. 2009;163(1):1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Ecker A, Bushell ES, Tewari R, Sinden RE. Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol Microbiol. 2008;70(1):209–220. doi: 10.1111/j.1365-2958.2008.06407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JW, et al. Loss-of-function analyses defines vital and redundant functions of the Plasmodium rhomboid protease family. Mol Microbiol. 2013;88(2):318–338. doi: 10.1111/mmi.12187. [DOI] [PubMed] [Google Scholar]
- 36.Hall N, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307(5706):82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 37.Lal K, et al. Characterisation of Plasmodium invasive organelles; an ookinete microneme proteome. Proteomics. 2009;9(5):1142–1151. doi: 10.1002/pmic.200800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patra KP, Johnson JR, Cantin GT, Yates JR, 3rd, Vinetz JM. Proteomic analysis of zygote and ookinete stages of the avian malaria parasite Plasmodium gallinaceum delineates the homologous proteomes of the lethal human malaria parasite Plasmodium falciparum. Proteomics. 2008;8(12):2492–2499. doi: 10.1002/pmic.200700727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linder ME, Deschenes RJ. Palmitoylation: Policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 40.Dighe SA, Kozminski KG. Swf1p, a member of the DHHC-CRD family of palmitoyltransferases, regulates the actin cytoskeleton and polarized secretion independently of its DHHC motif. Mol Biol Cell. 2008;19(10):4454–4468. doi: 10.1091/mbc.E08-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren J, et al. CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21(11):639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: Purification and identification. Nat Protoc. 2007;2(7):1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- 44.Khan SM, et al. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121(5):675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.