Significance
The number of species declines from the Earth's equator to the poles—a pattern known as the latitudinal diversity gradient (LDG)—for nearly every group of living organisms in which the LDG has been studied. However, our analyses of fossil occurrences demonstrate that the LDG of terrestrial mammals was substantially weaker than it is today, if not absent entirely, for most of the past 65 My. We also show the strength of the LDG varies with global temperature and is weakest during globally warm periods. We find that the modern LDG became established sometime in only in the last 4 My, most likely as a result of declining global temperatures.
Keywords: Mammalia, latitudinal diversity gradient, Cenozoic, climate, biogeography
Abstract
The decline of species richness from equator to pole, or latitudinal diversity gradient (LDG), is nearly universal among clades of living organisms, yet whether it was such a pervasive pattern in the geologic past remains uncertain. Here, we calculate the strength of the LDG for terrestrial mammals in North America over the past 65 My, using 27,903 fossil occurrences of Cenozoic terrestrial mammals from western North America downloaded from the Paleobiology Database. Accounting for temporal and spatial variation in sampling, the LDG was substantially weaker than it is today for most of the Cenozoic and the robust modern LDG of North American mammals evolved only over the last 4 My. The strength of the LDG correlates negatively with global temperature, suggesting a role of global climate patterns in the establishment and maintenance of the LDG for North American mammals.
The equator-to-pole decline in taxonomic richness, commonly referred to as the latitudinal diversity gradient (LDG) (although other terms, such as the latitudinal richness gradient are also used) is a pervasive biogeographic pattern of nearly every clade of extant organisms for which it has been studied (1–3). Despite its ubiquity and intense study, the ecological and evolutionary mechanisms underlying it remain uncertain, with a large number of potential causes that make similar predictions regarding the distribution of extant organisms (4, 5). The fossil record provides a distinct perspective from which to examine the LDG and can provide insights regarding mechanisms that might be inextricable using only extant taxa (e.g., refs. 6–9). For example, some proposed mechanisms to explain the modern LDG invoke particular climatic conditions that exist today, and the fossil record can be used to evaluate these mechanisms under different climatic conditions in the past (6, 10–15).
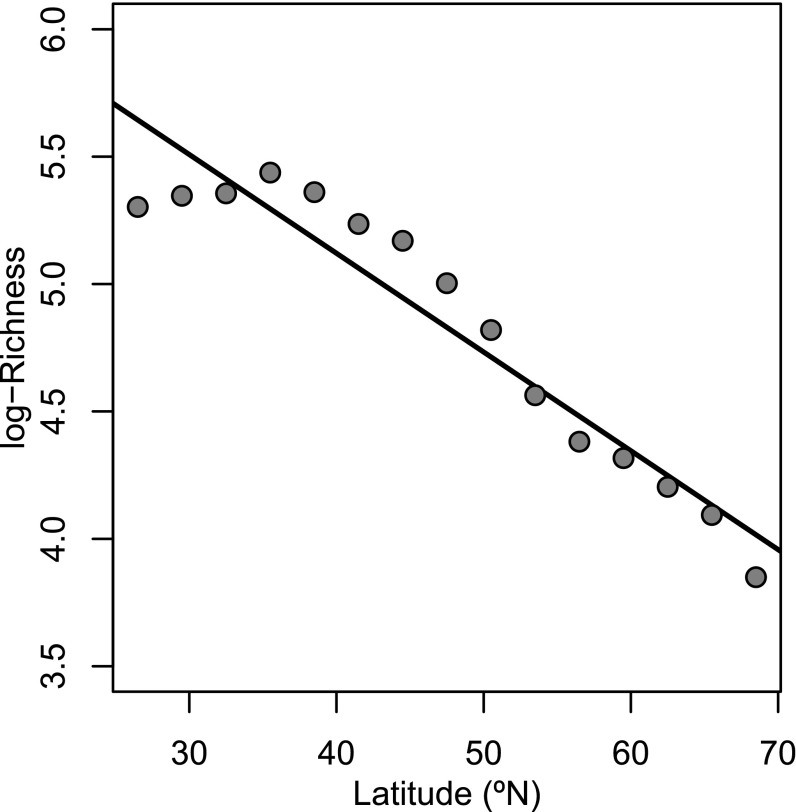
Modern North American mammals exhibit a typical LDG, with species richness declining substantially from low to high latitudes (Fig. 1 and refs. 16–20). However, we have shown previously (21) that the LDG for terrestrial North American mammals was absent during the mid-Paleocene Epoch (∼63–58 Ma). However, the latitudinal gradient in δ18O of Paleocene mammalian tooth enamel was similar to the modern latitudinal gradients in the oxygen isotopic composition of surface waters (precipitation, rivers, and lakes), which suggests that hydrological and climatic controls on δ18O gradients of surface waters were broadly similar during the Paleocene and today. The lack of a LDG in the Paleocene despite climatic gradients like those of today could be explained as a lingering result of the K/Pg extinction. In this scenario, the mechanisms underlying the modern LDG might have been operating in the Paleocene as they do today, but at that early stage in the postextinction radiation of mammals (22), sufficient time had not yet elapsed for the modern LDG to manifest at the continental scale. If so, a LDG like the modern should become established subsequent to the Paleocene, when North American mammalian faunas take on a modern taxonomic structure (e.g., refs. 23 and 24), then persist until today.
Fig. 1.
The modern mammalian LDG in North America. Extant species richness is pooled within latitudinal bands, 3° in width. Heavy black line shows the slope of the linear regression of the natural logarithm of species richness within each band on the latitudinal midpoint of each band.
Alternatively, the lack of a Paleocene LDG might reflect the fact that particular continental-scale aspects of climate (e.g., mean annual temperature, precipitation, seasonality, etc.) that drive the modern LDG (17) were at least qualitatively (e.g., spatially) different during the Paleocene. A recent review of studies of the LDG using the fossil record (6) summarizes the general influence of mean global temperature, showing that richness declines toward the poles only during intervals with relatively low global temperature (i.e., “icehouse worlds”), whereas globally warm intervals (i.e., “greenhouse worlds”) typically are characterized by a flat LDG or peak in taxonomic richness at temperate latitudes. Although the latitudinal gradient of temperature in western North America during the Paleocene was similar to today (21), mean global temperature was higher during the Paleocene than today (25), which might, in part, explain the lack of LDG. Mean global temperature has varied substantially since the Paleocene (25), but its relationship to the strength of the LDG over that interval remains unknown.
In the present study, we expand our perspective on the LDG to the entire Cenozoic history of terrestrial mammals in western North America. The fossil record of mammals in central and western North America is arguably the most densely and continuously sampled fossil record of any terrestrial group at the continental scale over the Cenozoic as a whole (26). As such, it is the most suitable—and probably the only—record with which to test the stability of the LDG of mammals at the continental scale over long expanses geological time. Previous studies of this record found variation in the strength of the relationships between latitude and both community composition and the spatial distribution of richness (i.e., β-diversity), respectively, over the Cenozoic (7). Here, we directly estimate the strength of the LDG in the past and demonstrate its variation over time.
To do so, we began by dividing the Cenozoic (2–65.5 Ma) into 32 time intervals of uniform 2-My duration. We then calculated the “face-value” pattern of richness across latitudes within each time interval, without accounting for variation in sampling among latitudes or intervals. In each interval, we determined species richness within latitudinal bands and intervals using fossil occurrences from the Paleobiology Database (https://www.paleobiodb.org; see Materials and Methods for details). We regressed richness on the midpoint latitudes of the bands to calculate the slope of the LDG (henceforth referred to as “face-value slope”).
Then, we used a conservative approach to determine and account for the influence on species richness of temporal and geographic variation of sampling (Materials and Methods). Intervals were retained for analysis only if they met two criteria: (i) at least three latitudinal bands had a minimum number (i.e., quota) of occurrences; and (ii) those latitudinal bands spanned at least 10° of latitude (Materials and Methods for full analytical details). Then, in each of these best-sampled time intervals that met these criteria, we used sample-standardization to estimate the relative richness among the included latitudinal bands only (i.e., latitudinal bands with fewer occurrences than the quota for the interval were not included for analysis). We regressed these subsampled richness estimates on the midpoint latitudes of the bands to calculate the slope of the LDG (henceforth referred to as “fossil slope”). We ultimately were able to calculate the fossil slope in 16 of 32 intervals that had sufficient sampling (henceforth referred to as “successful” intervals), including three separate stretches of time: (i) most of the Paleocene (four consecutive intervals), (ii) the Middle through Late Eocene (five consecutive intervals), and (iii) Middle Miocene through the Quaternary (seven consecutive intervals) (Table 1). Although restricting our analysis to just these successful intervals leaves many 2-My intervals unanalyzed here, our conservative approach ensures that our conclusions are based only on those intervals for which we could explicitly address sampling variation across latitudes, and the analyzed intervals collectively span the entire Cenozoic.
Table 1.
Summary statistics of North American mammalian LDG slopes over the Cenozoic
| Interval age, Ma | No. of successful reps (i.e., a slope for the fossil LDG was calculated) | Median (over reps) slope of the subsampled fossil LDG | Median (over reps) slope of the adjusted modern LDG | Proportion of successful reps in which fossil LDG was weaker than the adjusted modern LDG | Proportion of successful reps in which the fossil LDG was significantly different from the adjusted modern LDG (α = 0.05) | Proportion of successful reps in which the fossil slope was significantly greater than 0.0 (α = 0.05) |
| 2–4 | 6,280 | −0.020 | −0.031 | 0.762 | 0.058 | 0.100 |
| 4–6 | 1,940 | −0.001 | −0.038 | 0.994 | 0.502 | 0.097 |
| 6–8 | 14 | −0.001 | −0.039 | 1.000 | 0.929 | 0.143 |
| 8–10 | 8 | 0.005 | −0.036 | 1.000 | 0.875 | 0.250 |
| 10–12 | 113 | −0.001 | −0.055 | 1.000 | 0.451 | 0.062 |
| 12–14 | 148 | 0.017 | −0.037 | 1.000 | 0.757 | 0.176 |
| 14–16 | 107 | 0.034 | −0.033 | 1.000 | 0.935 | 0.729 |
| 16–18 | 0 | — | — | — | — | — |
| 18–20 | 0 | — | — | — | — | — |
| 20–22 | 0 | — | — | — | — | — |
| 22–24 | 0 | — | — | — | — | — |
| 24–26 | 0 | — | — | — | — | — |
| 26–28 | 0 | — | — | — | — | — |
| 28–30 | 0 | — | — | — | — | — |
| 30–32 | 0 | — | — | — | — | — |
| 32–34 | 0 | — | — | — | — | — |
| 34–36 | 0 | — | — | — | — | — |
| 36–38 | 78 | 0.003 | −0.023 | 0.949 | 0.718 | 0.282 |
| 38–40 | 3,074 | 0.015 | −0.025 | 0.949 | 0.480 | 0.158 |
| 40–42 | 2,220 | 0.021 | −0.021 | 0.989 | 0.782 | 0.441 |
| 42–44 | 2,401 | 0.021 | −0.023 | 1.000 | 0.815 | 0.463 |
| 44–46 | 2,334 | 0.021 | −0.023 | 1.000 | 0.823 | 0.454 |
| 46–48 | 0 | — | — | — | — | — |
| 48–50 | 0 | — | — | — | — | — |
| 50–52 | 0 | — | — | — | — | — |
| 52–54 | 0 | — | — | — | — | — |
| 54–56 | 0 | — | — | — | — | — |
| 56–58 | 0 | — | — | — | — | — |
| 58–60 | 186 | −0.010 | −0.101 | 1.000 | 1.000 | 0.414 |
| 60–62 | 339 | 0.003 | −0.092 | 1.000 | 1.000 | 0.386 |
| 62–64 | 4,647 | 0.017 | −0.057 | 0.991 | 0.701 | 0.194 |
| 64–66 | 4,122 | −0.038 | −0.057 | 0.856 | 0.391 | 0.654 |
Dashes indicate that sampling was insufficient to calculate numerical values. rep, pseudoreplicate analysis.
Results
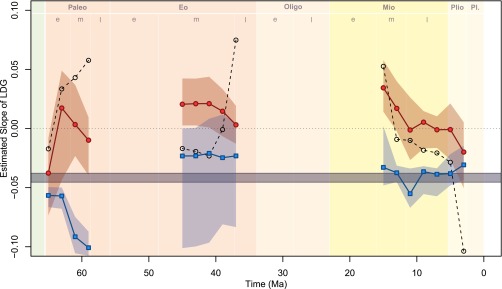
The slope of the LDG of North American terrestrial mammals varied substantially over the past 65 My (Fig. 2). Time series of face-value and sample-standardized fossil LDG slopes are broadly congruent (Fig. 2). Sample standardization appears to have reduced the overall variation of the estimated fossil LDG slopes across the entire Cenozoic, relative to face value, but has no other obvious pervasive effect. For example, sample standardization resulted in fossil slopes uniformly lower than face value during the Paleocene intervals but greater slopes in all successful Eocene intervals but one. In all successful intervals but one (the earliest Paleocene interval), the median fossil slope (over successful pseudoreplicate analyses) was greater than that of the modern North American mammalian LDG, which is strongly negative (i.e., indicating a decline in species richness from low to high latitudes) (Fig. 1). Furthermore, in all successful intervals but one, the calculated fossil slope was near or greater than 0.0, indicating a flat or reversed LDG throughout most of the Cenozoic.
Fig. 2.
The mammalian LDG in North America throughout the Cenozoic. LDG is quantified as the slope of the linear regression of the natural logarithm of species richness on latitude (degrees north of the equator). Points indicate the median slopes calculated within each 2-My interval, and the surrounding shading comprises the 95% CIs of slopes from the successful pseudoreplicate analyses (Materials and Methods). Red circles indicate sample-standardized estimates from the fossil record, and blue squares indicate the adjusted modern slope, estimated from extant species but constrained by the geographic distribution of fossil collections within that interval (Materials and Methods). The dashed line and open circles indicate the face-value (i.e., unstandardized) fossil LDG slopes. The horizontal gray bar shows the range of face-value estimates of the modern LDG slope when calculated using a range of latitudinal band widths (Materials and Methods).
To quantify the influence of the irregular geographic distribution of fossil collections on our estimates of the LDG, we calculated what the slope of the LDG using modern species would be if our knowledge of the geographic ranges of Recent species were restricted to the geography of fossil sampling within each interval (henceforth referred to as “adjusted modern LDG slope”; see Materials and Methods for details). The time series of adjusted modern LDG slopes indicate that the geographic distribution of fossil sampling does indeed influence the estimate of the LDG slope, but not to a great extent. In particular, the 95% confidence interval (CI) of the adjusted modern slopes were beyond the range of the estimates of the modern slope (see Materials and Methods for details) in only 5 of 16 successful intervals (Table 1) indicating that geographic variation in fossil sampling does not hopelessly obscure the fossil LDG. However, the 95% CI of fossil slopes overlaps the range of estimates of the modern slope in only four successful intervals (Fig. 2), further revealing that the terrestrial mammalian LDG substantially differed from the modern LDG for most of the Cenozoic.
In 15 of the 16 successful intervals, the fossil slopes were weaker (i.e., less negative) than the adjusted modern LDG slopes in more than 85% of the successful pseudoreplicate analyses (Table 1 and Materials and Methods). In 14 of those 15 intervals, the median fossil slopes lie beyond the 95% CI of the adjusted modern slopes (when median and CI are calculated over the successful pseudoreplicate analyses). Calculated another way, in each pseudoreplicate analysis, we determined whether the adjusted modern slope fell within the calculated 95% CI of the fossil using the regression of standardized fossil richness on latitude (see Materials and Methods for details). In 12 of the 16 successful intervals, the fossil slope was beyond this 95% CI of the adjusted modern slope in the majority (>50%) of the pseudoreplicate analyses (Table 1).
Discussion
Our results strongly demonstrate that the mammalian LDG of North America significantly differed from the modern LDG throughout most of the Cenozoic. Furthermore, the lack of a LDG previously observed in the mid-Paleocene (21) was not merely a lingering consequence of the K/Pg extinction and subsequent mammalian radiation but rather a persistent feature of mammalian biogeography in North America for most of the Cenozoic. The fossil slope is indistinguishable from the modern (negative) LDG during only three intervals: the earliest Paleocene (63–65 Ma), the late Eocene (36–38 Ma), and the Pliocene–Pleistocene (2–4 Ma). The modern-like LDG slope during the earliest Paleocene interval is surprising given the strong support for a lack of a LDG during the mid-Paleocene shown in this and our previous study (21), which used different approaches than we do here. Our result for the earliest Paleocene is puzzling in terms of the temporal and spatial proximity to the K/Pg impact event and associated mass extinction, and the Paleocene as a whole is not an interval of major global or regional climate change. During the late Eocene, the fossil slopes fall within the CI of the adjusted modern slope for one interval, which lies at the end of a gradual global cooling trend that lasted more than 10 My, and culminates in the abrupt climate change associated with the onset of Antarctic glaciation at the Eocene–Oligocene boundary (27). Finally, the fossil and modern slopes become indistinguishable in the most recent interval (2–4 Ma) in the Pliocene and early Pleistocene, suggesting that the modern LDG was established sometime in only the last 4 My.
We do not expect that taphonomic incompleteness would greatly distort the observed latitudinal patterns of taxonomic richness. Undoubtedly, the number of species preserved at a fossil locality is often a subset of those that actually inhabited the area in life. However, we pooled species richness among localities within latitudinal bands, so species that escaped sampling at one locality potentially could be found at another locality within the band. We used our sample-standardization procedure to equalize the number of analyzed occurrences among latitudes, thereby reducing the variation among latitudes of this probability of finding these missing species. Furthermore, we are not aware of any evidence for a persistent, systematic, continental-scale bias in taphonomic incompleteness throughout the Cenozoic that could account for the absent or reversed LDG slopes over the analyzed region in nearly every successful interval in our time series.
We restricted our geographic region of analysis to the most continuously and densely sampled region of western North America between 25° N and 70° N latitudes. There are few pre-Pleistocene collections (and constituent occurrences) beyond these geographic restrictions (e.g., in tropical Central America and eastern North America). Latitudinal bands including these collections beyond our latitudinal bounds would almost always be excluded from our analyses due to their small sample sizes (Materials and Methods). Because we did not consider fossil localities south of 25°N, we cannot rule out the possibility that tropical taxonomic richness was substantially higher than within our midlatitude focal region during the Cenozoic. Of the few fossil mammal localities south of 25°, most have very few (fewer than four) occurrences and/or are younger than the temporal window of this analysis. A few older collections (e.g., Arikareean Galliard Cut Fauna of Panama and the Hemphillian–Blancan Rancho el Ocote and Chadronian Iniyoo faunas from Mexico) consist of modest numbers of occurrences. Nevertheless, taxonomic richness of these collections is, at most, comparable with similarly aged faunas further north, and far below that observed for the same latitudes today (17). If there were substantially higher, although unsampled, tropical richness, it could only produce a disjunct latitudinal richness pattern in the Northern Hemisphere, consisting of a tropical peak paired with the observed flat gradient throughout midlatitudes. Such a pattern would still differ substantially from modern pattern, which is characterized by a strong gradient over the entire geographic range analyzed in this study, as demonstrated by the adjusted modern slopes.
Mannion et al. (6) classify hypotheses explaining the modern LDG into geographic, historical, and climatic themes. Geographic hypotheses generally attribute greater richness near the equator to the greater geographic area of the globe that is present at lower latitudes. Such hypotheses are not viable explanations for the modern LDG of terrestrial mammals in North America because land area generally decreases southward from North to Central America. Furthermore, the area of habitable North America has not changed over the Cenozoic to an extent that could have eliminated or reversed the LDG. Indeed, during the Plio–Pleistocene interval, in which the modern-like LDG appears, the extensive habitable terrestrial area of high-latitude North America would have disappeared episodically during major ice sheet advances.
The hypotheses under the historical theme generally attribute the lower richness at higher latitudes to a greater degree of climatic disruption in the geologic past, which caused frequent ecosystem upheaval, or even complete habitat loss, in the case of widespread high-latitude glaciations. At a global scale, such extreme climatic disruptions are characteristic of only the recent geological past. Northern hemisphere polar glaciations first occurred during the Late Miocene and intensified during the Pliocene (28), culminating in the cyclic Pleistocene glacial advances into central North America that recurred at Milankovitch band periodicities. Such dramatic climatic and environmental modifications coincide with the pronounced decline in LDG slope we observe between the most recent two analyzed intervals (4–6 and 2–4 Ma). Nevertheless, before the onset of persistent, cyclic, and extensive northern hemisphere high-latitude glaciations, we know of no obvious reason to expect environmental changes at higher latitudes of North America to have been any more drastic than those at low latitudes. Therefore, the historical hypotheses cannot account for the variation in LDG slope throughout most of our time series.
The timing of the development of the modern LDG of North American mammals coincides reasonably well with the completion of the Isthmus of Panama and the onset of the Great American Biotic Interchange (GABI), so it is reasonable to consider whether the appearance of the modern LDG was driven by immigration of South American lineages and their subsequent diversification in the context of the preexisting flat latitudinal diversity gradient. However, the fossil record documents only a modest number of immigrations into North America of species with South American origins (e.g., primates, marsupials, bats, xenarthrans, and a few species of rodents) as the isthmus developed during the late Cenozoic (29, 30). Given the standing diversity of endemic North American clades further north throughout the GABI, these immigrants were too few in number to tip a flat gradient into its modern configuration.
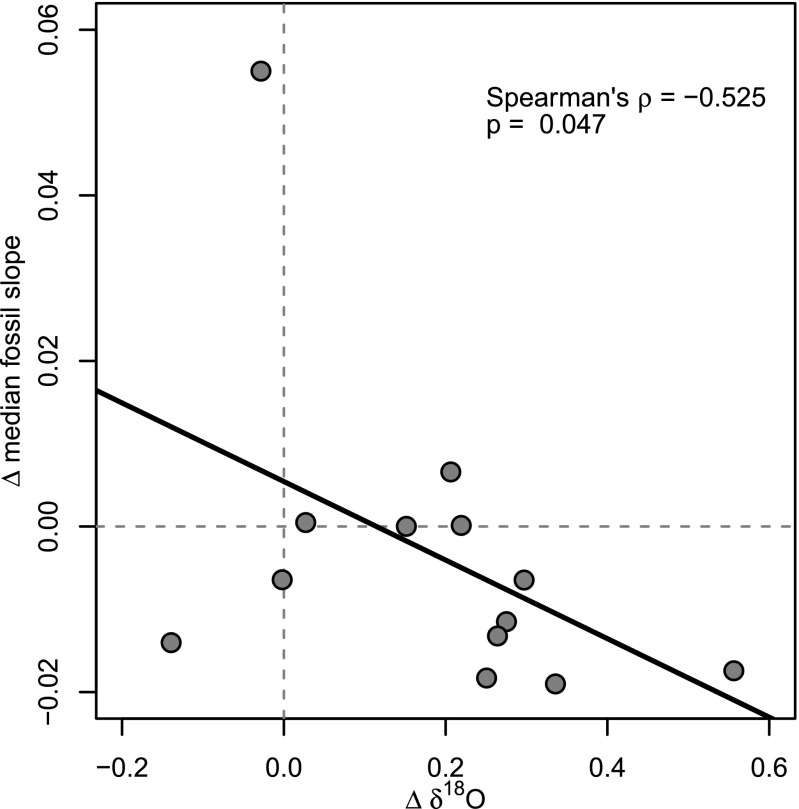
Finally, the climatic theme encompasses hypotheses directly or indirectly attributable to the lower seasonality and higher insolation found at lower latitudes. The weaker or even reversed LDG in the geologically recent past suggests that the latitudinal distribution of insolation cannot be its only causal mechanism. More generally, these hypotheses generally appeal to latitudinal temperature gradients, which are obvious today. During intervals of higher global temperature that characterize most of the Cenozoic, it is possible that such gradients were not as strong as today, and such variation might relate to the observed variation in LDG strength. Indeed, a perceptible influence of global temperature on the strength of the LDG is revealed by the statistically significant negative correlation between interval-to-interval changes (i.e., first-differences) of the median fossil slopes in each interval with first-differences of the mean δ18O values of benthic foraminifera from the same intervals (Fig. 3; Spearman’s ρ = −0.525; P = 0.047; benthic foraminifera data from ref. 25). In other words, when average global temperature (as measured by δ18O values of benthic foraminifera) increases from one interval to the next, the LDG weakens and vice versa. Although one coordinate pair appears to be an outlier high on the abscissa, we demonstrate that it does not have an undue influence on the strength of the correlation (SI Materials and Methods and Fig. S1). The strength of this correlation is weak, but all of the more compelling, given that the connection between δ18O values of benthic marine organisms and the biogeographic patterns of terrestrial mammals is so indirect (e.g., ref. 31). Nevertheless, it is plausible that global temperature modulates the strength of the LDG. Previous studies indicate a positive relationship between global biodiversity and global temperatures (32, 33). Furthermore, the maximum number of mammalian species that can inhabit a particular region is undoubtedly limited by restrictions on space and other resources. Today, because of their generally greater taxonomic richness, tropical regions are nearer this limit than temperate and polar regions, so the latter have more “ecological space” to accommodate additional species that are expected during warmer intervals. Indeed, warmer global temperatures during the Eocene led to subtropical environments and greater species richness of plants (34, 35), insects (36), and mammals (37–39) in temperate and polar regions than observed today. The disproportionate accommodation of species by temperate and polar regions during globally warm intervals would reduce the strength of the LDG. In this scenario, the strength of the LDG is not determined strictly by the climatic gradient from equator to pole but rather by the warmer temperature at high latitudes and concomitant intensification of productivity there. This hypothesis should be tested in the future with a more complete understanding of continental climates throughout the Cenozoic. Our analysis of the history of the LDG for North American mammals over the Cenozoic indicates that the three themes of explanatory factors for the modern LDG cannot be considered in isolation from each other. Instead, they have acted in concert dynamically over the course of the Cenozoic as global climate has changed from warmer, less volatile conditions in the early to middle Cenozoic to cooler, more volatile conditions since the Late Miocene.
Fig. 3.
Relationship between mean global temperature and the slope of the fossil LDG. Points represent changes between subsequent time intervals (i.e., first-differences) of mean oxygen isotope values [data are from Zachos et al. (25)] versus median slopes of the LDG using fossil data. The heavy black line represents least-squares linear regression.
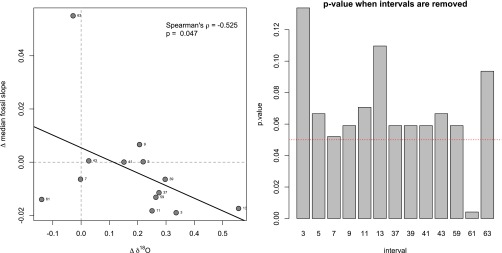
Fig. S1.
The dependence of the correlation between first-differences on each coordinate pair of δ18O and fossil slopes. (Left) The same as Fig. 3 but with the addition of labels adjacent to each point. This correlation is between first-differences: changes between consecutive intervals, so the point labels refer to the younger of the two intervals. For example, the point labeled “63” is the change in slope and δ18O between the interval beginning at 65 Ma and the subsequent interval beginning 63 Ma. (Right) Diagram showing the P value of the Spearman correlation when the indicated point is removed.
SI Materials and Methods
The reader understandably may be concerned that the strength of the observed correlation between first-differences of fossil slope and δ18O may be disproportionately influenced by what appears to be an outlier on the abscissa of Fig. 3 (figure repeated in Fig. S1, Left). First of all, we emphasize that we determined the strength of this correlation using Spearman’s nonparametric rank correlation. Therefore, the absolute value of the point showing the highest mean change in median fossil slope does not influence the strength of the correlation. In other words, if the value were 0.02 or 0.20—any value, so long as that point remained the highest observed value among all of the points—the strength of the nonparametric correlation would remain unchanged.
Nevertheless, to demonstrate that the apparent outlier does not solely determine the strength of the observed correlation between first-differences of fossil slope and δ18O, we repeatedly recalculated the correlation, each time excluding one pair of coordinates.
The results of this analysis are shown in Fig. S1. The removal of any point, with the exception of “61,” increases the P value greater than 0.05. Generally, this is simply the predicable result of losing statistical power with smaller sample sizes. Three points in particular (“3,” “13,” and “63”) seem to increase the P value substantially more than the others, one of these being the apparent outlier (“63”). Therefore, point “63” is not the only point that has a great influence on the strength of the correlation. Furthermore, removing the apparent outlier (“63”) results in only the third-greatest increase in P value, indicating that point “63” is not even the greatest contributor to the strength of the correlation. Notably, one point (“61”) actually decreases the P value substantially, and removal of this point would actually increase the strength of the correlation.
In summary, given our rigorous vetting of intervals for analysis (Materials and Methods), we prefer to use the data from all of available intervals and believe the use of nonparametric correlation helps ameliorate the distorting effects of potential outliers on the correlation.
Materials and Methods
Fossil Occurrence Data.
We obtained from the Paleobiology Database (https://www.paleobiodb.org) all mammalian occurrences from North America (i.e., Canada, Mexico, and the coterminous United States) over the Cenozoic (i.e., 2–65.5Ma) on May 21, 2015 (data were primarily derived from the North American Fossil Mammal Systematics Database; https://paleobiodb.org/cgi-bin/bridge.pl?page=OSA_3_North_American_mammals). We did not analyze the time interval younger than 2 Ma because Quaternary occurrences are much less completely represented in the Paleobiology Database than the remaining Cenozoic. We restricted our analysis to the well-sampled region between 25°N and 70°N latitude and 180° W and 90° W longitude. There are few collections (and constituent occurrences) beyond these geographic restrictions (e.g., in tropical Central America and eastern North America), and as such, latitudinal bands including these would be excluded from our analyses because of their small sample sizes (Sample Standardization). To restrict our focus to the terrestrial LDG, we excluded from analysis the following largely or exclusively marine groups: Cetacea, Sirenia, Desmostylia, Otariidae, Phocidae, and Odobenidae. After these geographic and taxonomic restrictions, 27,093 occurrences remained for analysis. The dataset used in this analysis is available in the Dryad Digital Repository (dx.doi.org/10.5061/dryad.53n6n).
Extant Geographic Range Maps.
We downloaded geographic range maps of 756 North American mammal species as shape files from NatureServe (40) on January 4, 2006. We imported these shape files into R for analysis using the R package rgdal. Scripts for this and all other analyses are available in the Dryad Digital Repository (dx.doi.org/10.5061/dryad.53n6n).
Fossil LDG.
To understand whether and how the mammalian LDG changed over the Cenozoic, we divided the time interval between 2 and 65.5 Ma into 32 time intervals (henceforth, “intervals”), each with a uniform 2-My duration, and determined the slope of the LDG in each. In each interval, we designated latitudinal bands (henceforth, “bands”) of equal latitudinal width (see Variation in Bandwidth below) and determined the number of unique species within each band using the paleolatitudes (i.e., the latitudes at the time of deposition) of the fossil occurrences. We then calculated the LDG slope in each interval as the linear regression of the natural logarithm of species richness within a band against the latitudinal midpoint (i.e., degrees north of the equator) of the band.
Our use of 2-My intervals necessarily bins taxa that were not coeval, thereby time-averaging the actual LDG. Nevertheless, we believe that binning taxa into time intervals makes our analysis more conservative. Our null hypothesis is a temporally constant LDG, with a slope equal to that observed today. Under this null hypothesis, pooling taxa over 2 My should only increase the apparent strength of the LDG.
Several other factors can bias our estimate of the true LDG slope including those particular to the fossil record (e.g., uncertainty in age of fossil collections, temporal and geographic variation in sampling) and parameters of the analysis (e.g., duration of intervals, widths of latitudinal bands). We attempted to ameliorate these potential biases using by performing 10,000 pseudoreplicate analyses, each of which analyzed identical occurrence data, but varied particular parameters using several procedures described below. Although these sources of bias present an analytical challenge (e.g., ref. 7), we calculate the LDG only when a strict set of criteria are met (see Sample Standardization below) and therefore only report the most robust estimates of the LDG.
Uncertainty of collection ages.
Collections in the Paleobiology Database typically are assigned maximum and minimum absolute age estimates. (Any rare occurrences lacking minimum or maximum absolute age estimates were excluded from analysis.) In each of the 10,000 pseudoreplicate analyses, we assigned each collection an absolute age drawn from a random uniform distribution bounded by that collection’s designated minimum and maximum ages.
Variation in bandwidth.
Because of the irregular geographic distribution of fossil collections, the choice of bandwidth affects the calculated LDG slope. Accordingly, each pseudoreplicate analysis used a different bandwidth between 1° and 5° of latitude, uniformly incremented by 0.0004°. Ultimately, the influence of bandwidth on the estimated slope and the difference between the fossil and extant slopes was small (Fig. S2).
Fig. S2.
Calculated LDG slopes for all band widths for each analyzed interval. The calculated fossil (squares) and adjusted modern slopes (circles) are shown for each successful pseudoreplicate analysis, each of which had a different bandwidth (Materials and Methods). Pairs of points are colored (red for fossil slopes, blue for adjusted modern slopes) for pseudoreplicates in which the fossil slope was significantly greater than the adjusted modern slope (i.e., a weaker LDG). Pairs of points are both colored gray for pseudoreplicate analyses in which the slopes were not significantly different. Note that in some intervals, slopes could only be calculated (Materials and Methods for criteria for analysis) for a small subset of band widths (e.g., 11 Ma). Nevertheless, although the choice of bandwidth did influence the absolute value of the slopes calculated, it very rarely influenced the relationship between the fossil and adjusted modern slope.
Sample standardization.
Uneven sampling among latitudes could bias our estimate of the slope of the LDG from fossil data. For example, greater sampling at northern latitudes versus southern latitudes artificially could give the perception of higher richness at higher latitudes and, therefore, an inverse LDG. We attempted to standardize sampling among latitudinal bands within an interval by randomly drawing (without replacement) the same number of occurrences from each band (e.g., rarefaction of occurrences among bands). We set the quota for this subsampling as the maximum observed (i.e., face-value) species richness within any single band within the interval. We selected this value because any smaller quota necessarily would distort the pattern of species richness (i.e., the face-value richness could not be recovered). We excluded from the calculation of LDG slope any latitudinal bands with fewer occurrences than the quota.
For comparison, we also calculated the face value (i.e., unstandardized) slopes in each interval by performing the same pseudoreplicate analyses described above, except for the sample-standardization among bands.
Influence of geographic distribution of fossil collections on LDG slope.
Although the sample-standardization procedure described above equalized the number of occurrences drawn from each band, some bands still sampled a broader longitudinal range than others due to the irregular geographic distribution of fossil occurrences. Because of small sample sizes within bands, geographic standardization (e.g., ref. 41) of sampling among latitudinal bands was not feasible. In lieu of standardization, we quantified the degree to which this variation in longitudinal sampling might bias our estimate of the fossil LDG slope by determining its effect on the extant LDG slope. In other words, we determined how the modern LDG slope would be distorted if we were to sample extant mammals from only the geographic area circumscribing the fossil collections. We henceforth refer to this as the “adjusted modern LDG.”
Specifically, we reduced each band within an interval to a polygon bounded to the north and south by the extreme latitudes of the band, and the east and west by the by the longitudes of the westernmost and easternmost fossil collections found within that band. We then used the over() function in R package sp to determine which extant species geographic range maps overlapped these polygons and then pooled these to determine the extant species richness in each polygon. As with the fossil LDG slopes, we calculated the extant LDG slope as the linear regression of the natural logarithm of species richness within a polygon against the latitudinal midpoint (i.e., degrees north of the equator) of the polygon.
Pseudoreplicate success.
Ultimately, we calculated LDG slopes for a particular pseudoreplicate analysis only if subsampled fossil richness could be determined in three or more bands, and the latitudinal range of these bands spanned at least 10° of latitude. Because of the geographic distribution of fossil collections, these conditions were not met for all band widths in all intervals. As a result, there are nine intervals for which slopes could not be calculated in any pseudoreplicate analysis. In the remaining intervals, the slope of the LDG could be calculated in only a subset, ranging from 8 to 6,280, of the 10,000 pseudoreplicate analyses (Table 1).
Modern LDG.
For comparison with the results of the previously described analyses, we used the extant geographic range maps to estimate the face-value slope of the modern LDG of North American mammals, irrespective of the distribution of fossil collections. As above, we used the over() function in R package sp to determine which species geographic range maps overlapped the same bands described above, only here, not limiting the longitudinal range. We then pooled these to determine the extant species richness in each band. As above, we calculated the extant LDG slope as the linear regression of the natural logarithm of species richness within a band against the latitudinal midpoint (i.e., degrees north of the equator) of the band. Bandwidth had little influence on the resulting face-value slopes, which ranged between −0.0454 and −0.0378 (Fig. 2).
Acknowledgments
Conversations with M. Kosnik, S. Lyons, S. Peters, and P. Wagner greatly improved the analytical design of this study. Portions of this work were supported by National Science Foundation Grant EAR 1252123 (to J.D.M.). This work is Paleobiology Database Publication 263.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.P. is a guest editor invited by the Editorial Board.
Data deposition: Scripts and the dataset used for the analyses reported in this paper have been deposited in the Dryad Digital Repository (dx.doi.org/10.5061/dryad.53n6n).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524750113/-/DCSupplemental.
References
- 1.Willig MR, Kaufman DM, Stevens RD. Latitudinal gradients of biodiversity: Pattern, process, scale and synthesis. Annu Rev Ecol Syst. 2003;34:273–309. [Google Scholar]
- 2.Hillebrand H. On the generality of the latitudinal diversity gradient. Am Nat. 2004;163(2):192–211. doi: 10.1086/381004. [DOI] [PubMed] [Google Scholar]
- 3.Gaston KJ. Global patterns in biodiversity. Nature. 2000;405(6783):220–227. doi: 10.1038/35012228. [DOI] [PubMed] [Google Scholar]
- 4.Rohde K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos. 1992;65(3):514–527. [Google Scholar]
- 5.Mittelbach GG, et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol Lett. 2007;10(4):315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 6.Mannion PD, Upchurch P, Benson RBJ, Goswami A. The latitudinal biodiversity gradient through deep time. Trends Ecol Evol. 2014;29(1):42–50. doi: 10.1016/j.tree.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Fraser D, Hassall C, Gorelick R, Rybczynski N. Mean annual precipitation explains spatiotemporal patterns of Cenozoic mammal beta diversity and latitudinal diversity gradients in North America. PLoS One. 2014;9(9):e106499. doi: 10.1371/journal.pone.0106499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell Matthew G. The latitudinal diversity gradient of brachiopods over the past 530 million years. J Geol. 2009;117(6):585–594. [Google Scholar]
- 9.Jablonski D, et al. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc Natl Acad Sci USA. 2013;110(26):10487–10494. doi: 10.1073/pnas.1308997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell MG. Latitudinal diversity gradients for brachiopod genera during late Palaeozoic time: Links between climate, biogeography and evolutionary rates. Glob Ecol Biogeogr. 2007;16(4):519–528. [Google Scholar]
- 11.Davies TJ, Buckley LB, Grenyer R, Gittleman JL. The influence of past and present climate on the biogeography of modern mammal diversity. Philos Trans R Soc Lond B Biol Sci. 2011;366(1577):2526–2535. doi: 10.1098/rstb.2011.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romdal TS, Araújo MB, Rahbek C. Life on a tropical planet: Niche conservatism and the global diversity gradient. Glob Ecol Biogeogr. 2013;22(3):344–350. [Google Scholar]
- 13.Yasuhara M, Hunt G, Dowsett HJ, Robinson MM, Stoll DK. Latitudinal species diversity gradient of marine zooplankton for the last three million years. Ecol Lett. 2012;15(10):1174–1179. doi: 10.1111/j.1461-0248.2012.01828.x. [DOI] [PubMed] [Google Scholar]
- 14.Yasuhara M, Hunt G, Cronin TM, Okahashi H. Temporal latitudinal-gradient dynamics and tropical instability of deep-sea species diversity. Proc Natl Acad Sci USA. 2009;106(51):21717–21720. doi: 10.1073/pnas.0910935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Roy K, Jablonski D. Do past climate states influence diversity dynamics and the present-day latitudinal diversity gradient? Glob Ecol Biogeogr. 2014;23(5):530–540. [Google Scholar]
- 16.Kaufman DM. Diversity of New World mammals: Universality of the latitudinal gradients of species and bauplans. J Mammal. 1995;76(2):322–334. [Google Scholar]
- 17.Badgley C, Fox DL. Ecological biogeography of North American mammals: Species density and ecological structure in relation to environmental gradients. J Biogeogr. 2000;27(6):1437–1467. [Google Scholar]
- 18.Kilpatrick AM, Mitchell WA, Porter WP, Currie DJ. Testing a mechanistic explanation for the latitudinal gradient in mammalian species richness across North America. Evol Ecol Res. 2006;8:333–344. [Google Scholar]
- 19.McCoy ED, Connor EF. Latitudinal gradients in the species diversity of North American mammals. Evolution. 1980;34(1):193–203. doi: 10.1111/j.1558-5646.1980.tb04805.x. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JW. Analytical zoogeography of North American mammals. Evolution. 1974;28(1):124–140. doi: 10.1111/j.1558-5646.1974.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 21.Rose PJ, Fox DL, Marcot J, Badgley C. Flat latitudinal gradient in Paleocene mammal richness suggests decoupling of climate and biodiversity. Geology. 2011;39(2):163–166. [Google Scholar]
- 22.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48(1):107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 23.Janis CM. Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu Rev Ecol Syst. 1993;24:467–500. [Google Scholar]
- 24.Janis CM. Patterns in the eovlution of herbivory in large terrestrial mammals: The Paleogene of North America. In: Sues H-D, editor. Evolution of Herbivory in Terrestrial Vertebrates: Perspectives from the Fossil Record. Cambridge Univ Press; Cambridge, UK: 2000. pp. 168–222. [Google Scholar]
- 25.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 26.Alroy J. Speciation and extinction in the fossil record of North American mammals. In: Butlin RK, Bridle JR, Schluter D, editors. Speciation and Patterns of Diversity. Cambridge Univ Press; Cambridge, UK: 2009. [Google Scholar]
- 27.Pagani M, et al. The role of carbon dioxide during the onset of Antarctic glaciation. Science. 2011;334(6060):1261–1264. doi: 10.1126/science.1203909. [DOI] [PubMed] [Google Scholar]
- 28.Mudelsee M, Raymo ME. Slow dynamics of the Northern Hemisphere glaciation. Paleoceanography. 2005;20(4):PA4022. [Google Scholar]
- 29.Woodburne MO. The Great American Biotic Interchange: Dispersals, tectonics, climate, sea level and holding pens. J Mamm Evol. 2010;17(4):245–264. doi: 10.1007/s10914-010-9144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vázquez-Domínguez E, Arita HT. The Yucatan peninsula: Biogeographical history 65 million years in the making. Ecography. 2010;33(2):212–219. [Google Scholar]
- 31.Alroy J, Koch PL, Zachos JC. Global climate change and North American mammalian evolution. In: Erwin DH, Wing SL, editors. Deep Time: Paleobiology’s Perspective. The Paleontological Society; Lawrence, KS: 2000. pp. 259–288. [Google Scholar]
- 32.Erwin DH. Climate as a driver of evolutionary change. Curr Biol. 2009;19(14):R575–R583. doi: 10.1016/j.cub.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 33.Mayhew PJ, Bell MA, Benton TG, McGowan AJ. Biodiversity tracks temperature over time. Proc Natl Acad Sci USA. 2012;109(38):15141–15145. doi: 10.1073/pnas.1200844109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington GJ, Eberle J, Le-Page BA, Dawson M, Hutchison JH. Arctic plant diversity in the Early Eocene greenhouse. Proc Biol Sci. 2012;279(1733):1515–1521. doi: 10.1098/rspb.2011.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing SL. Tertiary vegetation of North America as a context for mammalian evolution. In: Janis CM, Scott KM, Jacobs LL, editors. Evolution of Tertiary Mammals of North America. Vol 1. Cambridge Univ Press; New York: 1998. pp. 37–65. [Google Scholar]
- 36.Archibald SB, Bossert WH, Greenwood DR, Farrell BD. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology. 2010;36(3):374–398. [Google Scholar]
- 37.Rybczynski N, et al. Mid-Pliocene warm-period deposits in the High Arctic yield insight into camel evolution. Nat Commun. 2013;4:1550. doi: 10.1038/ncomms2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eberle JJ, Greenwood DR. Life at the top of the greenhouse Eocene world–A review of the Eocene flora and vertebrate fauna from Canada’s High Arctic. Geol Soc Am Bull. 2011;124(1-2):3–23. [Google Scholar]
- 39.Eberle JJ, Rybczynski N, Greenwood DR. Early Eocene mammals from the Driftwood Creek beds, Driftwood Canyon Provincial Park, northern British Columbia. J Vertebr Paleontol. 2014;34(4):739–746. [Google Scholar]
- 40.Patterson BD, et al. 2003. Digital Distribution Maps of the Mammals of the Western Hemisphere, Version 3.0 (NatureServe, Arlington, VA)
- 41.Marcot JD. The fossil record and macroevolutionary history of North American ungulate mammals: Standardizing variation in intensity and geography of sampling. Paleobiology. 2014;40(2):238–255. [Google Scholar]