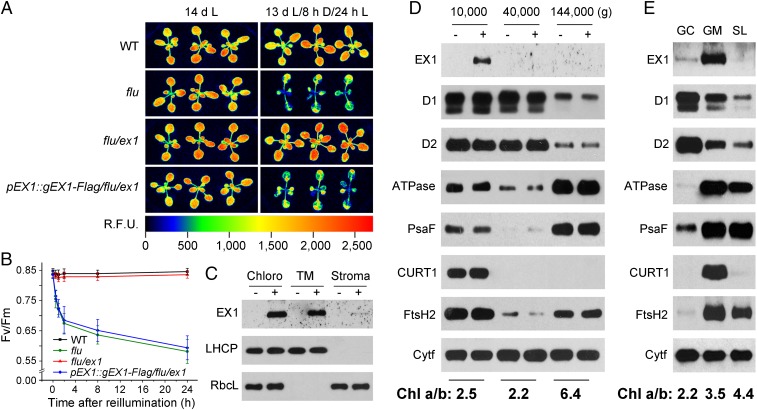
Fig. 1.
The biological activity and localization of EX1-FLAG. (A) Complementation of flu/ex1 with a genomic DNA construct that encodes EX1 fused to the FLAG-tag (gEX1-Flag) under control of the native EX1 promoter (pEX1). WT, flu, flu/ex1, and the complemented flu/ex1 line (pEX1::gEX1-Flag/flu/ex1) were grown for 13 d under continuous light and either kept for another 32 h under continuous light (14 dL) or transferred to the dark for 8 h and re-exposed to light for 24 h (13 dL/8 hD/24 hL). Singlet oxygen-mediated growth inhibition and photoinhibition of PSII as revealed by transient Chl fluorescence changes in flu are suppressed in flu/ex1 and restored in the complemented flu/ex1 line. R.F.U.: Relative Fluorescence Unit. (B) Singlet oxygen-mediated changes of the maximum efficiency of PSII (Fv/Fm). Seedlings were grown for 5 d under continuous light, shifted to the dark for 8 h, and re-exposed to light for various lengths of time. PSII activity is inhibited in flu and the complemented flu/ex1 but not in flu/ex1 and WT, indicating that 1O2-mediated and EX1-dependent signaling occurs under nonphotoinhibitory light and 1O2 generated in seedlings with a flu background does not directly damage PSII. The results represent the mean and SD of Fv/Fm measurements of at least 30 individual seedlings. (C) Localization of EX1-FLAG in chloroplast membranes. Chloroplasts (Chloro) isolated from flu/ex1 complemented with pEX1::gEX1-Flag (+) and noncomplemented flu/ex1 control lines (−) were lysed and separated by centrifugation into membrane (TM) and stroma (Stroma) fractions. Proteins were solubilized, separated by SDS/PAGE, and analyzed on Western blots using antisera against FLAG, LHCP, and the large subunit of ribulose-1,5-bisphospate carboxylase (RbcL). (D) Localization of EX1-FLAG in grana stacks that retain their margin parts. Chloroplast membranes were treated with the detergent digitonin and separated by differential centrifugation into three subfractions that were pelleted at 10,000 × g, 40,000 × g and 144,000 × g. Proteins of these subfractions were analyzed by immunoblotting as shown in C, and the PSII RC proteins D1 and D2, the PSI-specific PsaF, ATPase, FtsH2, and the margin-specific CURT1 were used as markers to locate EX1-FLAG. Colocalization of EX1-FLAG and CURT1 indicates that EX1 is highly enriched in grana stacks that retain their margin parts. Cytochrome f (Cytf) was used as a loading control. (E) Localization of EX1-FLAG in grana margins. Detergent-treated chloroplast membranes were separated into grana core (GC), grana margins (GM), and stroma lamellae (SL). The same marker proteins as shown in D were used to locate EX1-FLAG. The various subfractions were also characterized by their Chl a/b ratios shown below D and E.