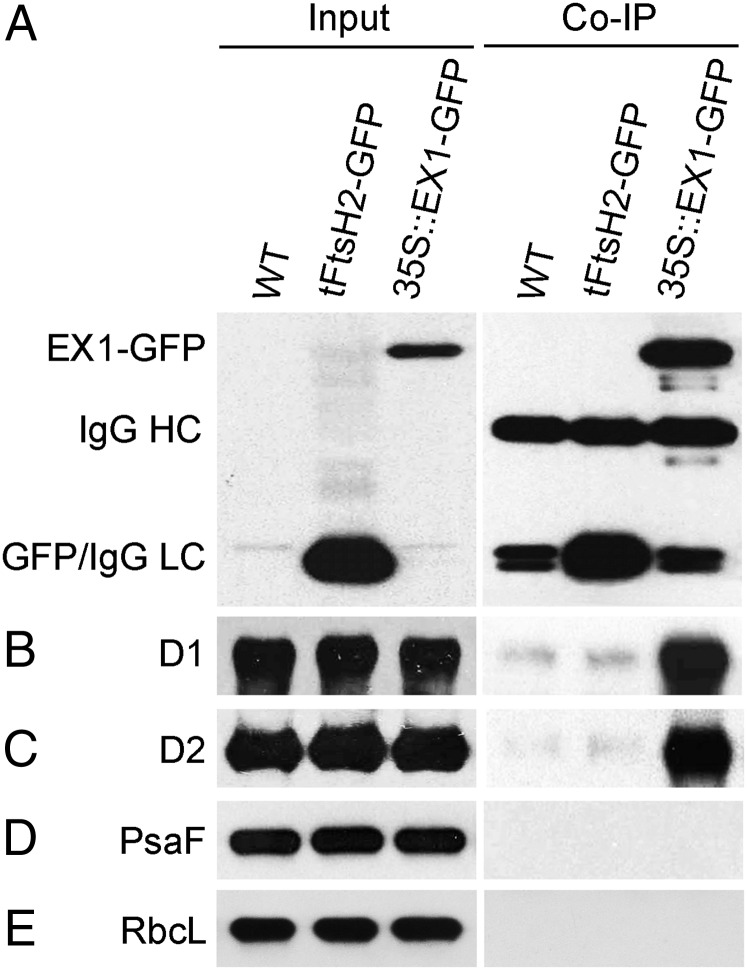
Fig. 2.
Identification of EX1-interacting proteins by coimmunoprecipitation. Input sample: Total proteins extracted from 5-d-old light-grown seedlings of WT and lines that express the plastid-targeted tFtsH2-GFP or EX1-GFP were separated by SDS/PAGE and probed on Western blots with antisera against GFP (A), D1 (B), D2 (C), PsaF (D), and RbcL (E). Co-IP sample: Immunoreactive proteins were precipitated from each of the three input samples by adding protein G beads with a conjugated monoclonal anti-GFP antibody. Bound proteins were solubilized and separated by SDS/PAGE and probed on Western blots as described above. The three unspecific bands cross-reacting with the GFP antibody in the WT immunoprecipitate sample represent the heavy (IgGHC) and two light (IgGLC) chains of IgG. In the tFtsH2-GFP immunoprecipitate, the IgGLC bands are superimposed by GFP. In the EX1-GFP immunoprecipitate sample, only EX1-GFP but not GFP is found. The PSII RC proteins D1 and D2 coimmunoprecipitate with EX1-GFP but not GFP or the PSI-specific PsaF. RbcL served as a nonbinding control.