Figure 3. Activation of the insulin‐signalling cascade in skeletal muscle .
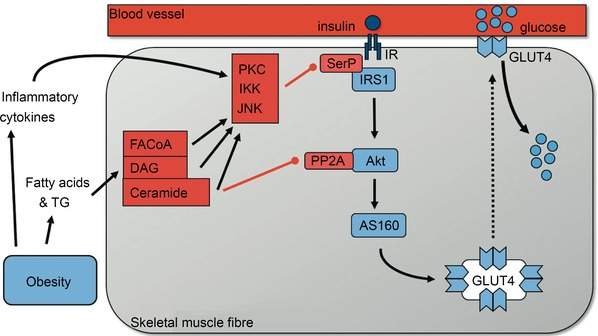
In healthy trained individuals increases in the insulin concentration in the interstitial fluid surrounding muscle fibres leads to increased activation of the insulin signalling cascade and translocation of GLUT4 (main glucose transporter in muscle) to the plasma membrane. As muscle is the main tissue responsible for glucose uptake in the period after meal ingestion this will keep the rise in blood glucose concentration following ingestion of a meal relatively modest. As such healthy trained individuals have firm control on their blood glucose levels, both in the fasted period and after food intake. In sedentary, obese or elderly individuals, and those with metabolic syndrome and type 2 diabetes, high blood levels of fatty acids and triglycerides contribute to an excessive accumulation of lipid metabolites such as long chain fatty acyl‐CoA (FACoA), diacylglycerol (DAG) and ceramide in skeletal muscle. This together with increased plasma levels of inflammatory cytokines in obese individuals (Berg & Scherer, 2005) and local inflammation of the microvasculature in skeletal muscle activates the serine kinases protein kinase C (PKC), IκB kinase (IKK) and Jun amino‐terminal kinase (JNK) (Wagenmakers et al. 2006). These phosphorylate insulin receptor substrate 1 (IRS1) on serine residues SerP leading to inactivation of IRS1 and downstream inactivation of the insulin signalling cascade. Ceramide accumulation also activates the phosphatase PP2A which dephosphorylates and inactivates Akt. These mechanisms collectively prevent activation of the insulin signalling cascade in skeletal muscle and therefore reduce insulin‐mediated activation of Akt substrate of 160 kDa (AS160) and GLUT4 translocation and skeletal muscle glucose uptake. Reproduced with permission from Shaw & Wagenmakers (2012), The Biochemist, © the Biochemical Society.
