FIGURE 8.
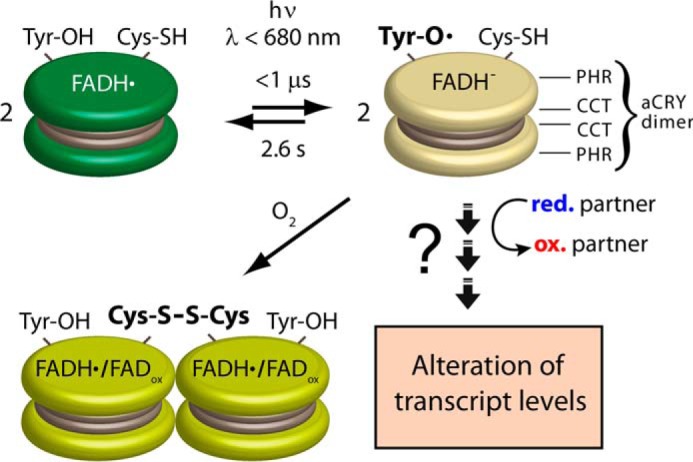
Model of the response to red light of aCRY in vitro. aCRY forms a homodimer (represented by two disks) via its CCT. aCRY carrying FADH• is reduced to FADH− by light with λ < 680 nm. As a result, TyrO• is formed by Tyr-373 within 1 μs with a red-shifted absorption in aCRY compared with water. The lifetime of 2.6 s of TyrO• is unusually high and might allow for an efficient oxidation of a signaling partner in vivo finally leading to the changes in the levels of multiple transcripts. The decay of TyrO• proceeds concomitant with that of FADH−. Some of the light-activated aCRY oligomerizes via disulfide bridge formation at Cys-482 in the absence of external reductant.
