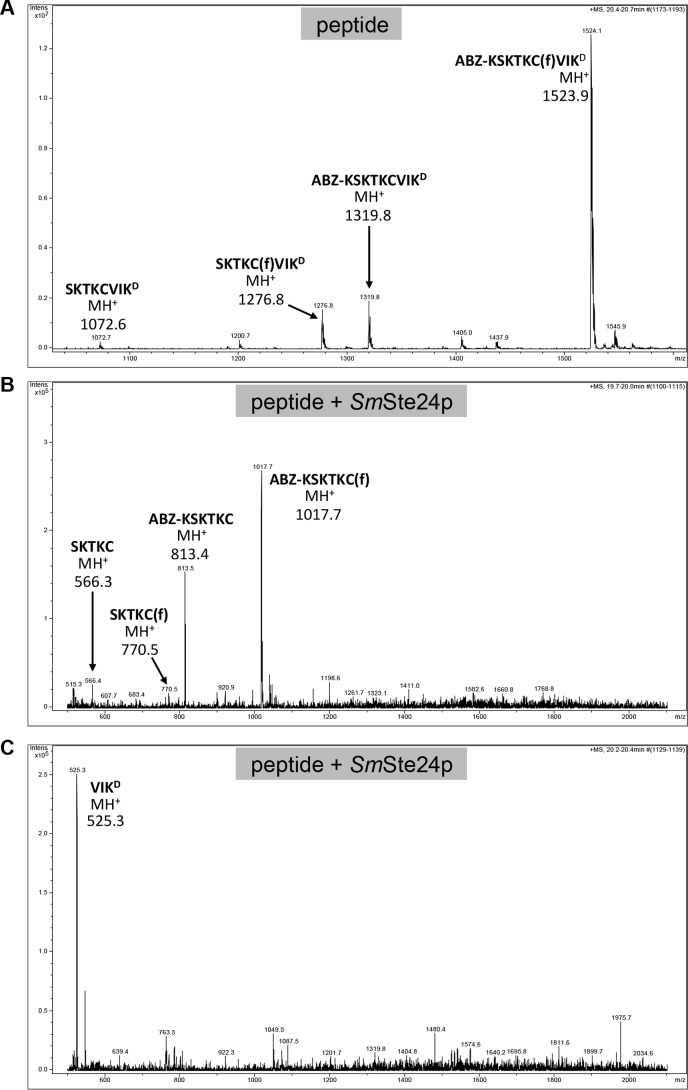
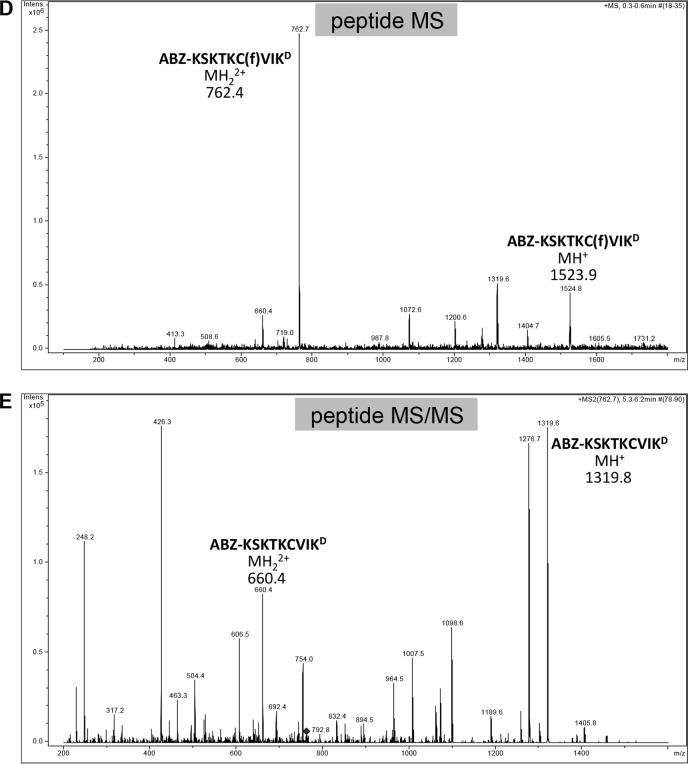
FIGURE 10.
Mass spectra of products derived from the K-Ras4b reporter treated with purified SmSte24p. A–C, samples of K-Ras4b reporter incubated in the absence (A) and presence of purified SmSte24p (B and C) were analyzed by LC-MS using a Bruker Esquire 3000 Plus mass spectrometer. The sequences and theoretical masses that correspond to identified peaks are shown centered or adjacent to appropriate peaks. One of the prenylated species in A lacking ABZ and N-terminal Lys (SKTKC(f)VIKD) is probably a minor impurity from peptide synthesis. Spectra for B and C were derived from different LC fractions corresponding to 19.7–20 min and 20.2–20.4 min, respectively. D and E, the non-prenylated species detected in A was not present in mass spectra provided by the peptide manufacturer; they are probably a by-product of ionization (67). In support of this, we observed that MS/MS analysis of the double ion form of the uncleaved farnesylated peptide (m/z 762.4) resulted in the loss of farnesylated peptide ions (MH+, m/z 1523.9; MH22+, m/z 762.4 m/z) (D) and the appearance of non-prenylated peptide ions (MH+, m/z 1319.8; MH22+, m/z 660.4) (E).