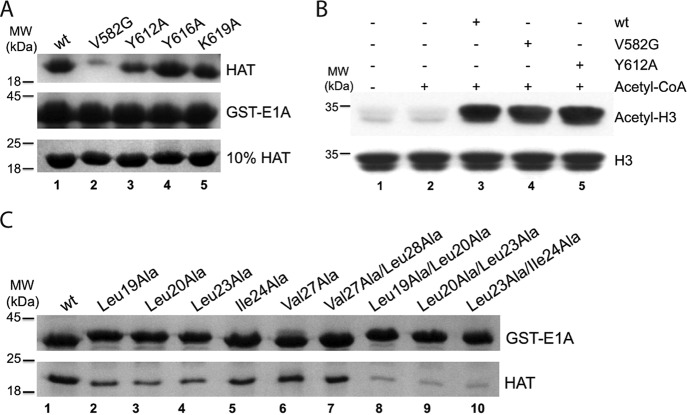
FIGURE 3.
Key residues in KAT2B and E1A interaction. A, disruptive mutations in the KAT2B HAT domain. The GST-E1A (1–126 aa) pulldown experiment was performed with 3.0 μm KAT2B HAT domains (wt and mutants). The bound KAT2B proteins are shown on the top gel and inputs are on the bottom gel. GST-E1A loading controls are shown on the middle gel. B, acetyltransferase activity of KAT2B mutants. H3 alone (lane 1), and further mixed with 20 μm acetyl-CoA without KAT2B (lane 2) were loaded as controls. C, disruptive E1A mutations for KAT2B interaction. GST pulldown experiment was performed with equal amounts of GST-E1A (1–126 aa) fusion protein and its mutants bound on GST beads (upper band) to pulldown the KAT2B HAT domain (lower band).