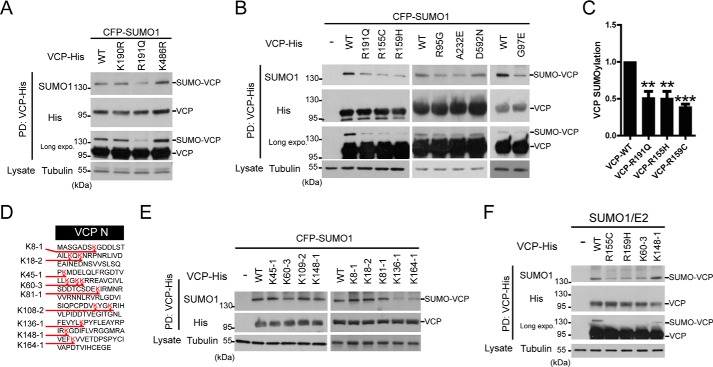
FIGURE 2.
Pathogenic mutations in the N-domain of VCP inhibit SUMOylation. A and B, the pathogenic mutation reduces the SUMOylation of VCP. HEK293 cells were co-transfected with the WT or indicated mutant VCP-His constructs together with SUMO-related plasmids, and analyzed for SUMO conjugation as described. C, the quantification of SUMOylation levels of indicated VCP constructs relative to that of the WT VCP in B. Data are shown as mean ± S.E., n = 3 experiments. D, protein sequence of the VCP N-domain with the sites of lysine (K) to arginine (R) mutations indicated. Every Lys in the N-domain was mutated and the position and the number of Lys to Arg mutations in each construct indicated (e.g. K60–3 harbors 3 Lys to Arg mutations starting at the Lys60). E, mutagenesis and SUMOylation analysis indicates 3 likely sites of SUMO conjugation in the N domain of VCP. All the Lys to Arg VCP mutant constructs were analyzed. F, the reduction of VCP SUMOylation by the pathogenic mutant is comparable with that of the SUMO-deficient mutant K60–3. The SUMO-insensitive K148 mutant was included as a control. HEK293 cells were transfected as indicated, followed by VCP SUMOylation analysis.