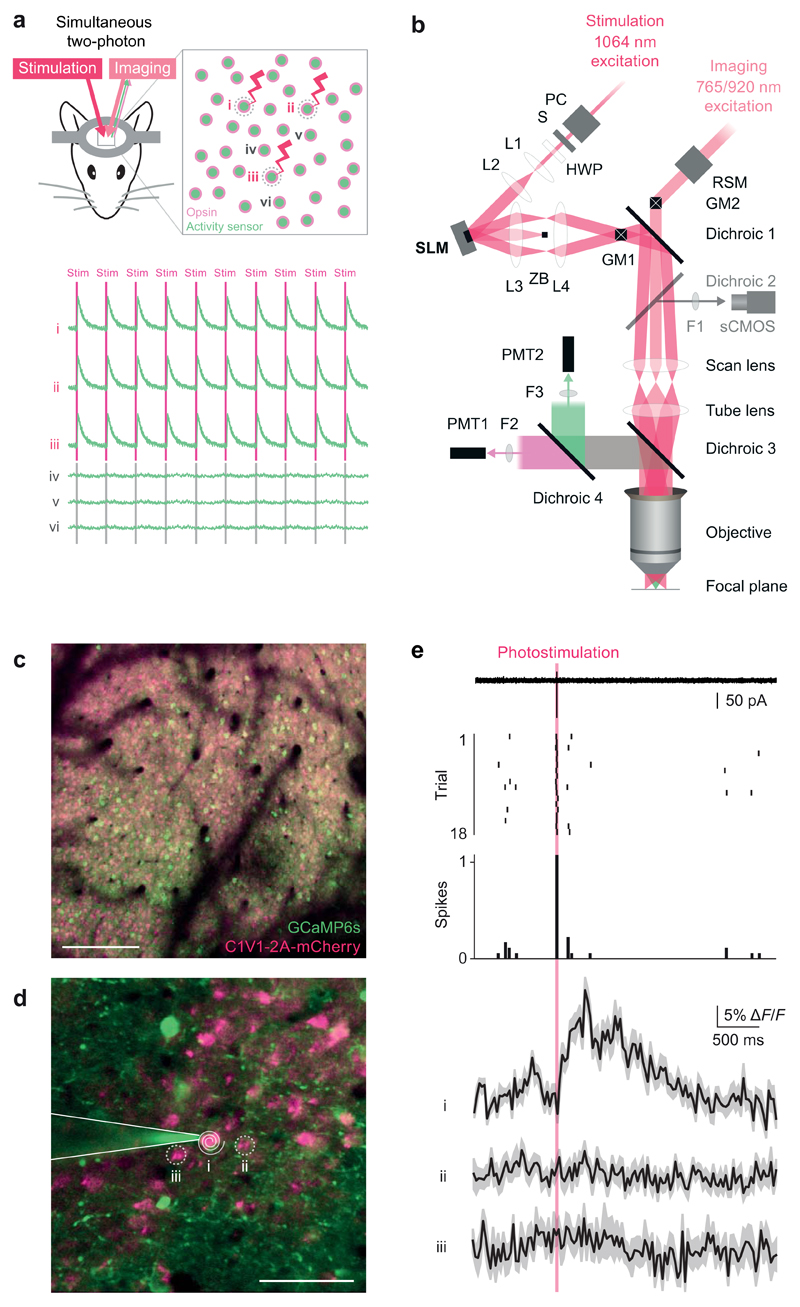
Figure 1. Single-cell two-photon optogenetic photostimulation and single action potential readout in vivo.
(a) Schematic illustration of the experimental goal. Successful interrogation of neural circuits at the resolution and precision at which they function requires a method with the specificity to simultaneously manipulate and record individual, user-selected neurons in the awake behaving animal. An all-optical approach to perform such experiments would be beneficial given the chronic and non-invasive nature in addition to the ability to localize neuron’s locations precisely in circuits where physical topology is critical. Top: Our approach utilizes a coexpression strategy to imbue neurons with read and write abilities: a calcium sensor generates an optical readout of activity while an opsin enables photostimulation. Bottom: The experimental goal would realize robust and reliable photostimulation in the user-selected neurons 1, 2, and 3 with sufficient resolution to avoid stimulating the immediately adjacent neurons 4, 5, and 6.
(b) Optical layout of the SLM-based (spatial light modulator) two-photon patterned photostimulation, two-photon resonant-scanning, moving in vivo microscope. The photostimulation beam (300 fs, 5 W, 1064 nm pulsed laser, Fianium Ltd or 100 fs, 2.3 W, 1055 nm pulsed laser, Coherent) was controlled by a Pockels’ cell (PC, Conoptics Ltd.) and shutter (S, Vincent Associates), resized using a beam-expanding telescope (L1 [f = 50 mm] and L2 [f = 200 mm], plano-convex lenses, Thorlabs) to fill the SLM active area and polarization maximized for diffraction efficiency from the SLM with a half wave plate (HWP, WPH10M-1064, Thorlabs). SLM: reflective spatial light modulator, 7.68 mm × 7.68 mm active area, 512 × 512 pixels, (Boulder Nonlinear Systems). L3: 1” achromatic doublet, f = 400 or 250 mm. ZB: zero order block. L4: 2” achromatic doublet, f = 150 mm. GM1: Galvanometer mirror pair, 3 or 6 mm (Cambridge Technology, integrated into dual beam Ultima microscope by Bruker Corp. [formerly Prairie Technologies]). GM2: Galvanometer mirror pair, 6mm (Cambridge Technology, integrated by Bruker Corp.). The imaging beam path can also be scanned along the fast axis by a resonant scanning galvanometer mirror (RSM, Cambridge Technology, integrated by Bruker Corp.) relayed onto GM2. Dichroic 1: 1030 nm short pass (T1030SP, Chroma Technology). Dichroic 2: 660 nm long pass (660LP, Chroma). This dichroic is used to image SLM beamlets in widefield mode on the sCMOS camera only when dichroic 3 is removed. F1: 675/67 nm bandpass filter (Semrock). sCMOS camera: ORCA-Flash4.0 (Hamamatsu). Scan lens: f=75 mm. Tube lens f=180 mm. Dichroic 3: 700 nm long pass (T700lpxxr-xxt, Chroma). Dichroic 4: 575LP (HQ575dcxr, Chroma). F2: 525/70 nm bandpass filter (525/70m-2P, Chroma). F3: 607/45 nm bandpass filter (607/45m-2P, Chroma). PMT1: Multi-alkali photomultiplier tube (Hamamatsu). PMT2: GaAsP photomultiplier tube (Hamamatsu). Objective: 16X 0.8 NA (Nikon).
(c) A large field of view of neurons co-expressing GCaMP6s and C1V1 (scale bar, 100 μm).
(d) Inset from a large field of view (200 × 200 μm) for the experiment shown in (e) (scale bar, 50 μm). A two-photon targeted cell-attached patch clamp recording was obtained from neuron 1, which co-expressed GCaMP6s and C1V1. This neuron was targeted for optogenetic stimulation by a beamspot produced by the SLM that was then driven in a spiral pattern by the galvanometer mirrors (white spiral).
(e) Top: electrophysiological recording during photostimulation trials reveals reliable generation of single action potentials during the photostimulation period (red bar) as evidenced in the single sweep (from trial 2), raster plot, and peristimulus time histogram. Bottom: calcium imaging recordings obtained simultaneously showed a transient (mean ± SEM matching the amplitude and kinetics expected for a single action potential with GCaMP6s in the photostimulated neuron, while the nearby neurons showed no detectable transients. Similar results were seen in n = 3 neurons.