FIGURE 5.
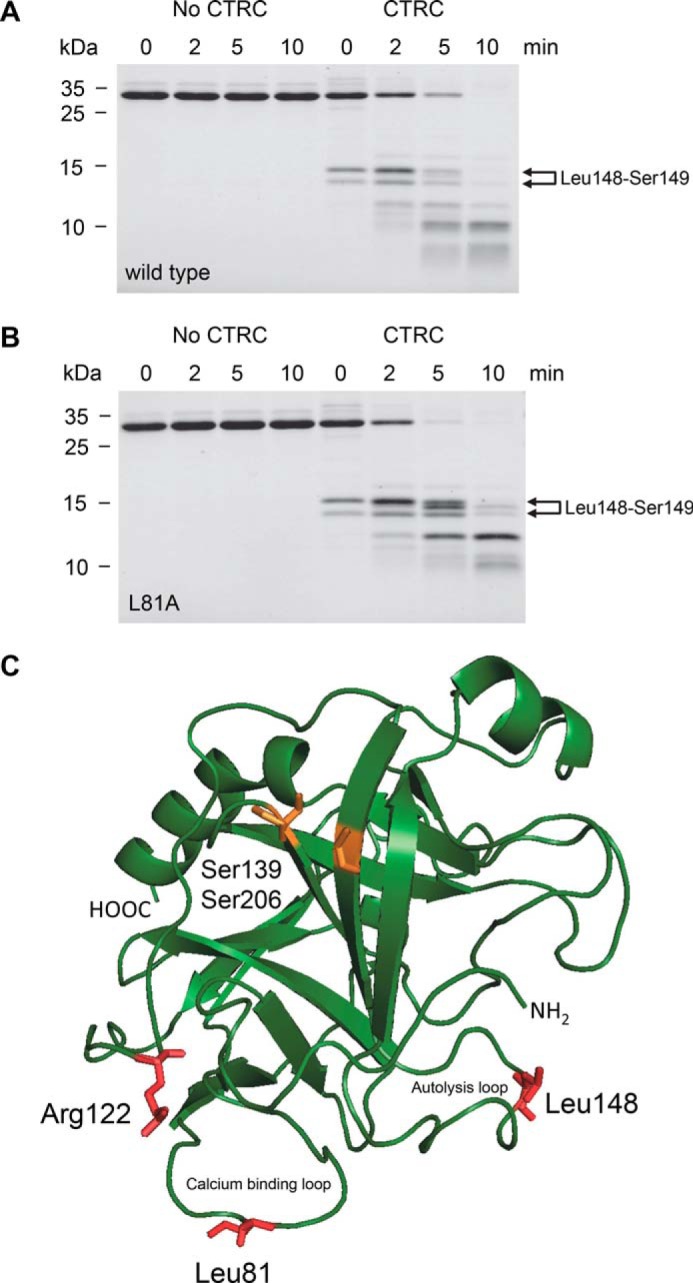
Cleavage of human anionic trypsinogen by CTRC. Wild-type (A) and L81A mutant (B) anionic trypsinogen (1 μm) was incubated at 37 °C with 10 nm CTRC in 0.1 m Tris-HCl (pH 8.0), 25 mm NaCl, and 20 nm human SPINK1 trypsin inhibitor (final concentrations). Trypsin inhibitor was included to prevent autoactivation during the cleavage reaction. At the indicated times, trypsinogen (150 μl) was precipitated with 10% trichloroacetic acid (final concentration) and analyzed by reducing SDS-PAGE and Coomassie Blue staining. The two bands generated by cleavage of the Leu-148–Ser-149 peptide bond are indicated. Note that the N-terminal upper band slightly shifts over time as the activation peptide is processed at Phe-18 by CTRC. See text for details. See “Experimental Procedures” for statement on experimental uncertainty and reproducibility. C, model of human anionic trypsinogen with regulatory cleavage sites indicated in red. The position of the missing disulfide replaced by Ser-139 and Ser-206 is highlighted in orange. Bovine cationic trypsinogen (Protein Data Bank file 1TGN (34)) was used as template to generate a model for anionic trypsinogen using the SWISS-MODEL structure homology-modeling server (35). The image was rendered by PyMOL 3.1.
