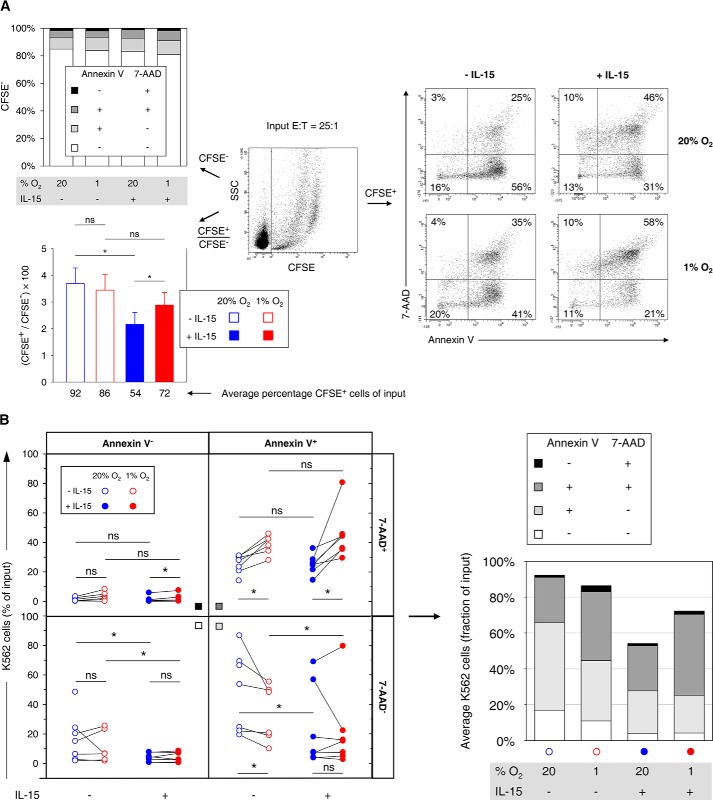
FIGURE 11.
Effects of hypoxia and IL-15 priming during NK cell conditioning on stages of target cell apoptosis. Effector NK cells (E) isolated from buffy coats were conditioned by preculture at 20 or 1% O2 for 22 h without or with IL-15 (45 ng/ml) present during the final 6 h, mixed, and co-incubated with CFSE labeled K562 target cells (T) at an E/T ratio of 25:1 for 4 h under normoxic standard culture conditions. Seven buffy coats were processed in independent experiments out of which one non-primed hypoxia sample was not available for apoptosis analysis. A, representative flow plot (center) shows differentiation of CFSE− effector and CFSE+ target cells. Stages of apoptosis were determined by multicolor flow cytometry in two-factor analysis with annexin V-APC/7-AAD staining as exemplified for target cells (right). The top left diagram summarizes mean proportions of viable (annexin V− 7-AAD−), early apoptotic (annexin V+ 7-AAD−), late apoptotic (annexin V+ 7-AAD+), and necrotic (annexin V− 7-AAD+) effector cells for different preculture conditions. The bottom left diagram displays average CFSE+/CFSE− ratios ± S.E. for each effector cell preculture condition from which average target cell fractions of input cell numbers were calculated on the basis of the E/T ratio of 25:1. B, differential effector cell conditioning caused redistributions of target cells among stages of apoptosis. Changes by hypoxia are highlighted by connecting paired data points, i.e. results from the same buffy coat (left). A stacked bar chart summarizes the distributions (right, cf. A, bottom left). *, p < 0.05; ns, not significant.