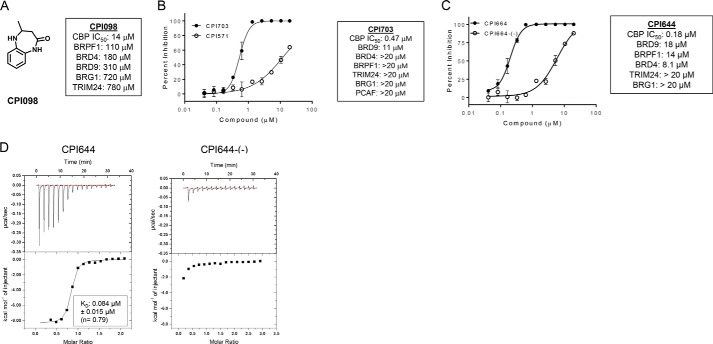
FIGURE 1.
Biochemical description and characterization of CBP/EP300 probe molecules. A, structure of CPI098 and summary table indicating potency against other bromodomains. B, CBP inhibition by CPI703 and CPI571. Representative AlphaLISA data are shown for each compound (duplicate, ± S.E.). Across multiple replicates CPI703 (closed circles) inhibits with an IC50 = 0.47 ± 0.07 μm (n = 4), and CPI571 (open circles) has an IC50 = 12.2 ± 0.4 μm (n = 3) (values ± S.E.). C, CBP inhibition by CPI644 and CPI644-(−). Representative AlphaLISA data is shown for each compound (duplicate, ± S.E.). Across multiple replicates CPI644 inhibits with IC50 = 0.18 ± 0.06 μm (n = 5) and CPI644-(−) (open circles) with IC50 = 6.0 ± 0.6 μm (n = 3) (values ± S.E.). D, ITC analysis with CPI644 or CPI644-(−) and the bromodomain of CBP. A binding stoichiometrically of n = 0.79 and a KD of 0.084 μm was observed for CPI644, although no detectable binding was observed for CPI644-(−).