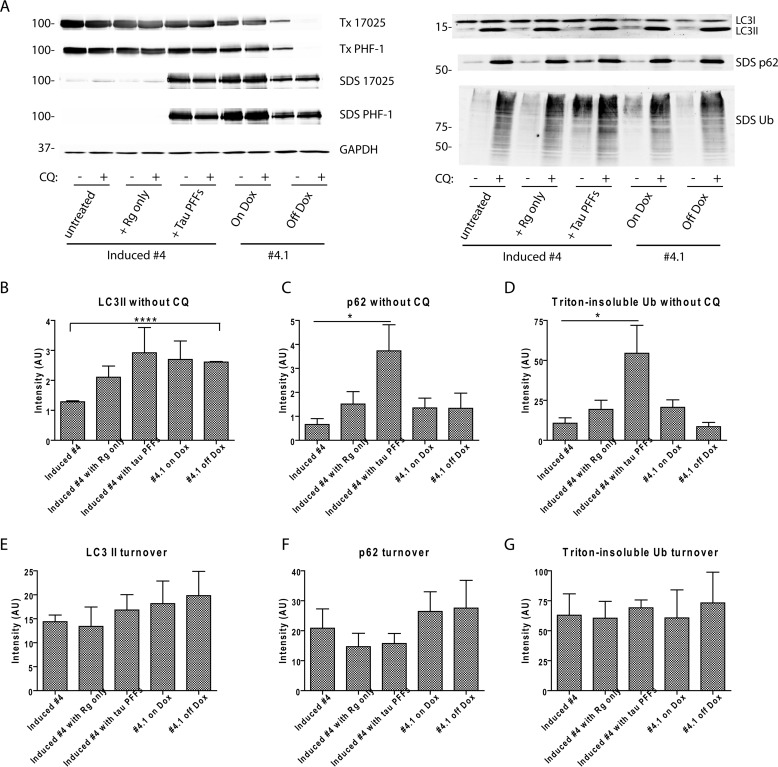
FIGURE 9.
Tau aggregation does not lead to significant impairment of autophagy flux. A, autophagy flux assay was conducted on 5 groups of cells: 1) induced clone 4 cells, 2) induced clone 4 cells newly treated with BioPORTER reagent, 3) induced clone 4 cells newly transduced with tau PFFs mediated by BioPORTER reagent, 4) clone 4.1 cells maintained on Dox, and 5) clone 4.1 cells withdrawn from Dox for 5 days. Levels of autophagy substrates (LC3-II, p62, and Triton (Tx)-insoluble ubiquitinated proteins) were compared for each group of cells with and without 2 days treatment of 30 μm CQ that blocked lysosomal degradation. Although LC3-II was completely extracted by 1% Triton X-100 lysis buffer, p62 was predominantly found in the SDS fraction. Differential solubility of tau in these 5 groups of cells was confirmed by immunoblotting with 17025 and PHF-1. GAPDH served as loading control. Immunoblots from one representative set of experiment were shown. B–D, densitometry quantification of LC3-II, p62, and Triton-insoluble ubiquitinated proteins for the experimental conditions shown. E–G, turnover of the three types of autophagy substrates during the 2-day period was estimated by (level with CQ treatment − level without CQ treatment). For B–G, quantification was based on 4 independent sets of experiment, except for n = 3 for clone 4.1 cells off Dox. Data are shown as mean ± S.E. *, p < 0.05. ****, p < 0.0000005.