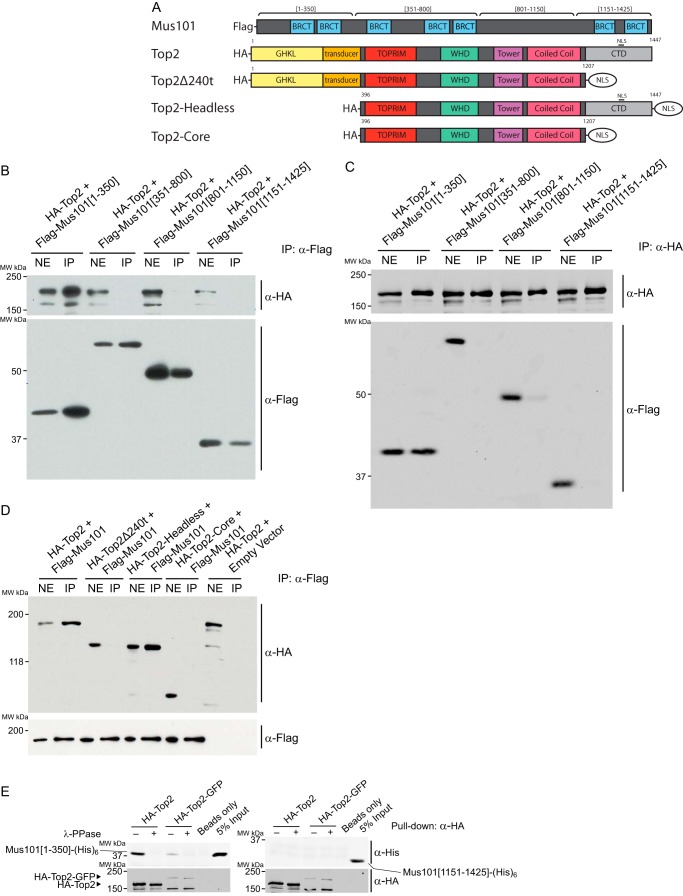
FIGURE 2.
Top2-Mus101 interaction requires the N-terminal domain of Mus101 and the C-terminal regulatory domain of Top2. A, schematic of the expression constructs for Mus101 and the HA-tagged Top2. GHKL, gyrase, Hsp90, histidine kinase, MutL, TOPRIM, topoisomerase-primase; WHD, winged helix domain; NLS, nuclear localization signal. B and C, S2 cells were transfected with ectopic expression vectors for HA-Top2 along with one of the FLAG-tagged Mus101 truncations, aa 1–350, aa 351–800, aa 801–1150, or aa 1151–1425. The nuclear extracts (NE) were prepared from lysed cells and then subjected to immunoprecipitation using anti-FLAG-agarose beads (B) or anti-HA-agarose beads (C). Products of IP were analyzed by immunoblot and detected by rabbit anti-FLAG antibodies for Mus101 fragments and mouse anti-HA antibodies for Top2. Mus101[1–350] is the only fragment that was immunoprecipitated with Top2. MW, molecular weight. D, similar immunoprecipitation experiments were carried out using nuclear extracts from S2 cells expressing FLAG-Mus101 and one of the following Top2 constructs: full-length Top2 (HA-Top2), C terminus-truncated Top2 (HA-Top2Δ240t), N terminus-truncated Top2 (HA-Top2-Headless), or Top2 with truncation at both termini (HA-Top2-Core). HA-Top2 and HA-Top2-headless can immunoprecipitate with FLAG-Mus101. E, 2 pmol of Mus101[1–350]-His6 or Mus101[1151-End]-His6 were tested in a pulldown experiment under the same conditions. HA-Top2 can associate with Mus101[1–350]-His6 but not with Mus101[1151-End]-His6.